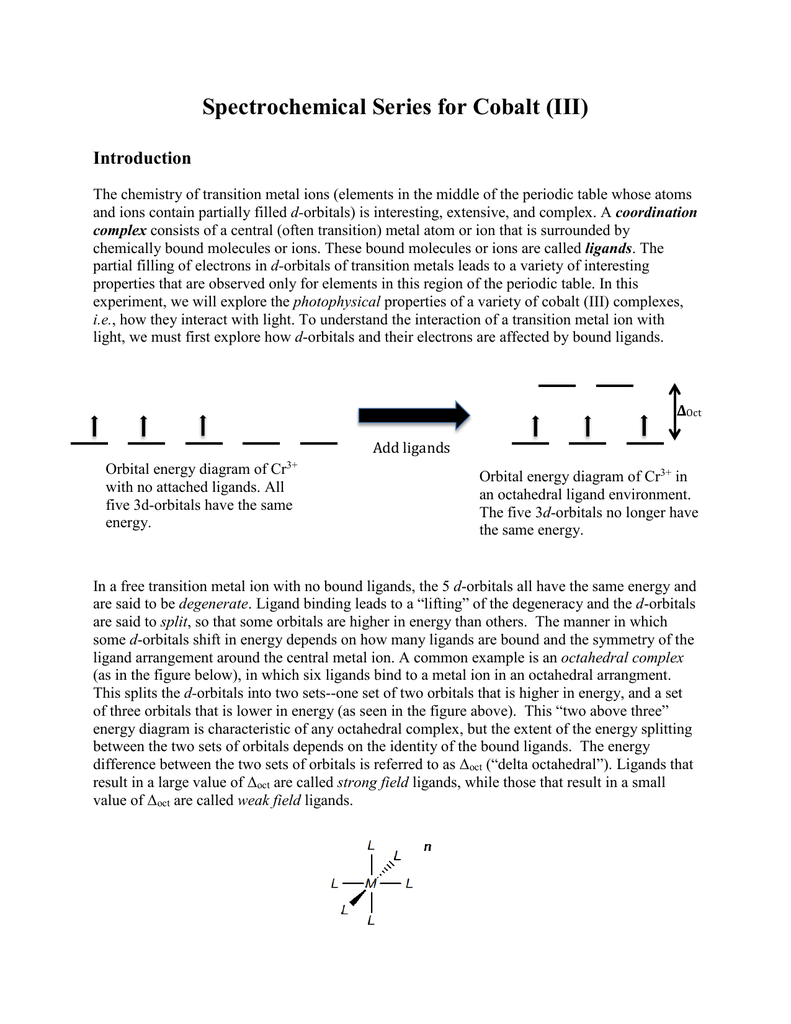
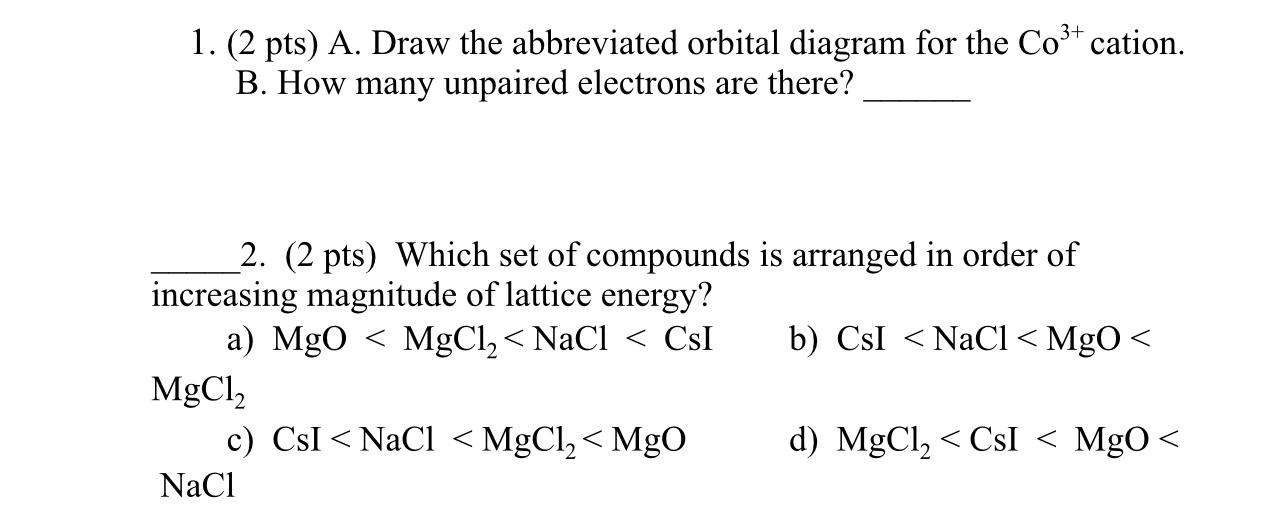
40 co3+ orbital diagram
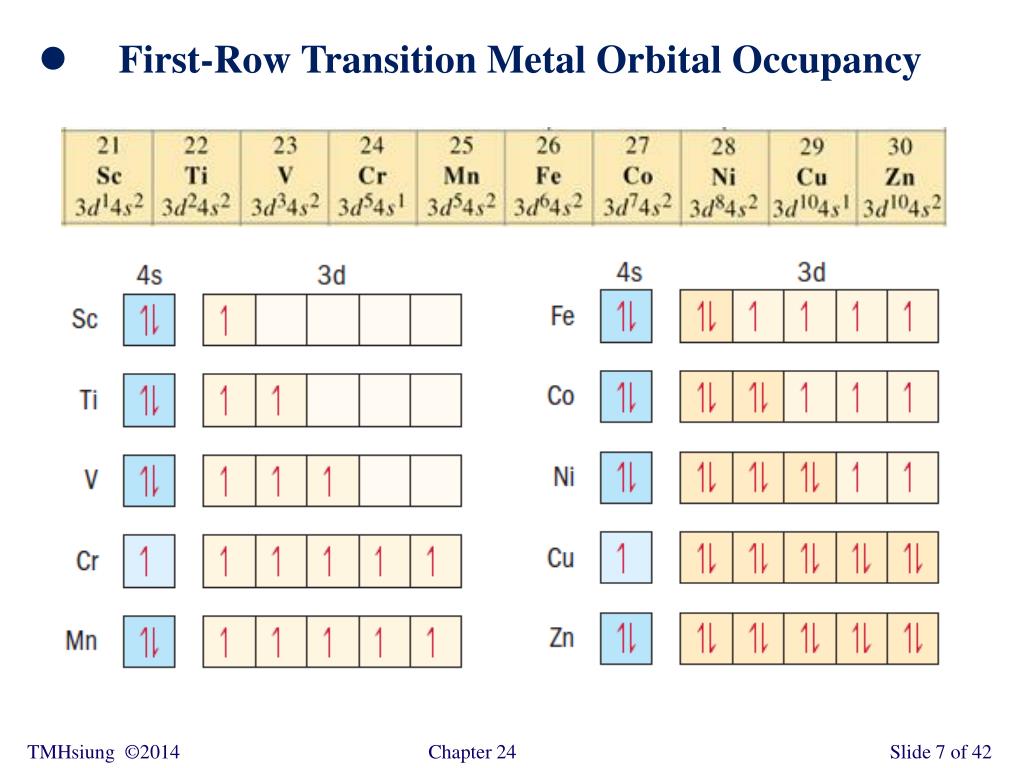
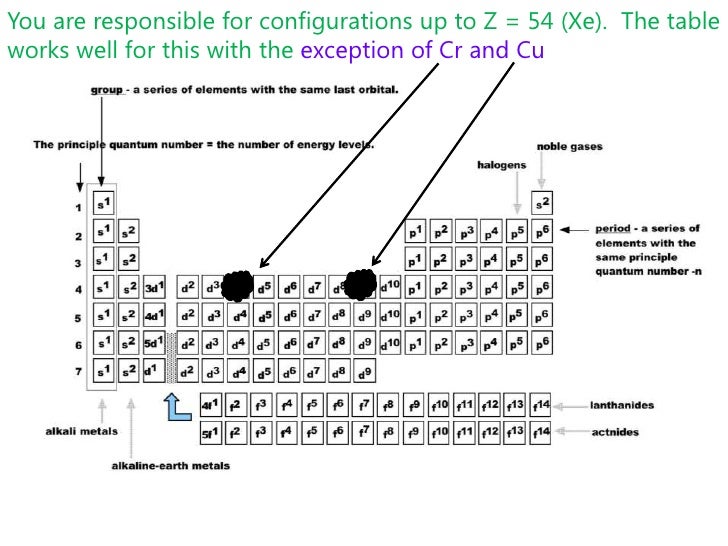
Does co3 have a pi bond? - Carbonate ion has one carbon atom, three oxygen atoms and -2 charge. One unhybridized 2p orbital of carbon will laterally overlap with one unhybridized 2p orbital of oxygen to form pi-bond. Thus, there is a presence of one double bond in the carbonate ion. Does ozone have delocalized pi bonds? The number of electrons in each of cobalts shells is 2 8 15 2 and its electron configuration is Ar 3d 7 4s 2 The cobalt atom has a radius of 125 pm and a Van der Waals radius of 192 pm. Give the complete electron configuration of cobalt Co and the complete or abbreviated electron configuration of Co2 and Co3.
Answer (1 of 2): In strong field delta0 will be more. Co =3d74s2 Co +3= 3d6 CN- is considered as strong ligand, hence all 6 electrons will be filled in t2g orbitals. All paired. Diamagnetic, inner orbital complex, d2sp3. Now in case of Br- is considered as weak ligand hence all 6 electrons wi...

Co3+ orbital diagram
CO32- Molecular Orbital (MO) Diagram What is MO theory? Molecular Orbital Theory is a concept of quantum mechanics that is used to decipher the chemical bonding nature inside different molecular structures. This is a complex yet useful tool that helps in sketching MO diagrams for better understanding. A gaseous Co3+ ion in the ground state has four unpaired electrons. In the same manner how many unpaired electrons are in an atom of carbon in its ground state? By Hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2 , is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ... The electron in its 1s orbital is shared with the Carbon atom. The carbon atom, which is also the central atom, is the one that requires hybridized orbitals to accommodate the shared electrons. We can find out the hybridization of the atom by using some simple and quick methods such as Steric Number and Number of sigma bonds.
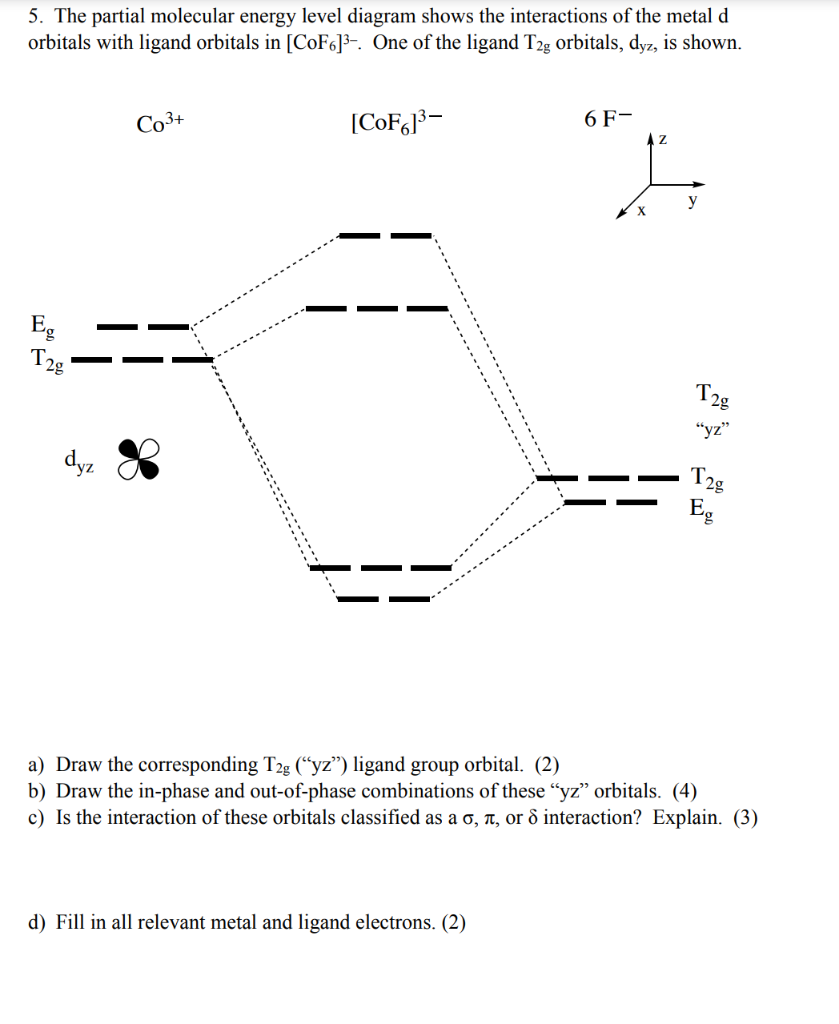
Co3+ orbital diagram. The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration. Learn more about the definition of the ground state electron ... O. diagram for [Co(NH3)6]3+ Dr. Mithil Fal Desai Shree Mallikarjun and Shri Chetan Manju Desai College Canacona Goa 2. t* 1u a1g t2g, eg a1g, t1u, eg a1g t1u a* 1g e* g eg t1u Δo t2g Metal (Ti3+)orbitals Co3+→[Ar] 3d6, 4s0 6e- Ligand group (NH3) orbitals 6 x 2 = 12 e- σ [Co(NH3)6]3+ molecular orbitals M. O. diagram for [Co(NH3)6]3+ complex ... Cobalt has a total of 27 electrons which are contained in 1s 2s 2p 3s 3p 4s and 3d sub. Orbital diagram of cobalt an orbital diagram is a way of showing where and how many electrons are in an atom of a certain element. The orbital diagram for the atom of cobalt is shown below. 1s2 2s2 2p1 boron 1s2 2s2 2p6 3s2 3p6 4s2 3d1. MO Diagram of H2CO3. The sigma bonds between C and O atoms are formed by the 2sp2 orbital of C and O atoms. It results in sigma bonding and antibonding orbitals. The sigma bond between oxygen and hydrogen use 1s orbital of hydrogen and 2sp3 orbitals of oxygen. It forms a sigma bonding and antibonding orbital.
The molecular orbital diagram of BeCl2 will be drawn by combining atomic orbitals of beryllium atom and group orbitals of chlorine atom having similar energy and symmetry around a molecular axis. The 3s group orbitals of chlorine atom will remain non-bonding because their energy is very low as compared to the 2s and 2p atomic orbitals of ... The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. The Lewis structure of sulfur trioxide (SO3) molecule is drawn by: First, look for the total number of valence electrons in a single sulfur trioxide (SO3) molecule, which is twenty-four. One unhybridized 2p orbital of carbon will laterally overlap with one unhybridized 2p orbital of oxygen to form pi-bond. Thus, there is a presence of one double bond in the carbonate ion. - Therefore, the carbon atom in carbonate ion, CO2−3 is sp2 hybridized.. What is the hybridization of carbon in CO3 2 −?, The central carbon atom is attached which double bonds with oxygen. Thus, pi electrons get shifted to terminal electronegative atom causes it renders a vacant orbital with central atom because a Lewis base (OH-) can easily donate lone pair of electrons to the central atom (C). SO2 and CO2 are the best examples of these types of Lewis acids.
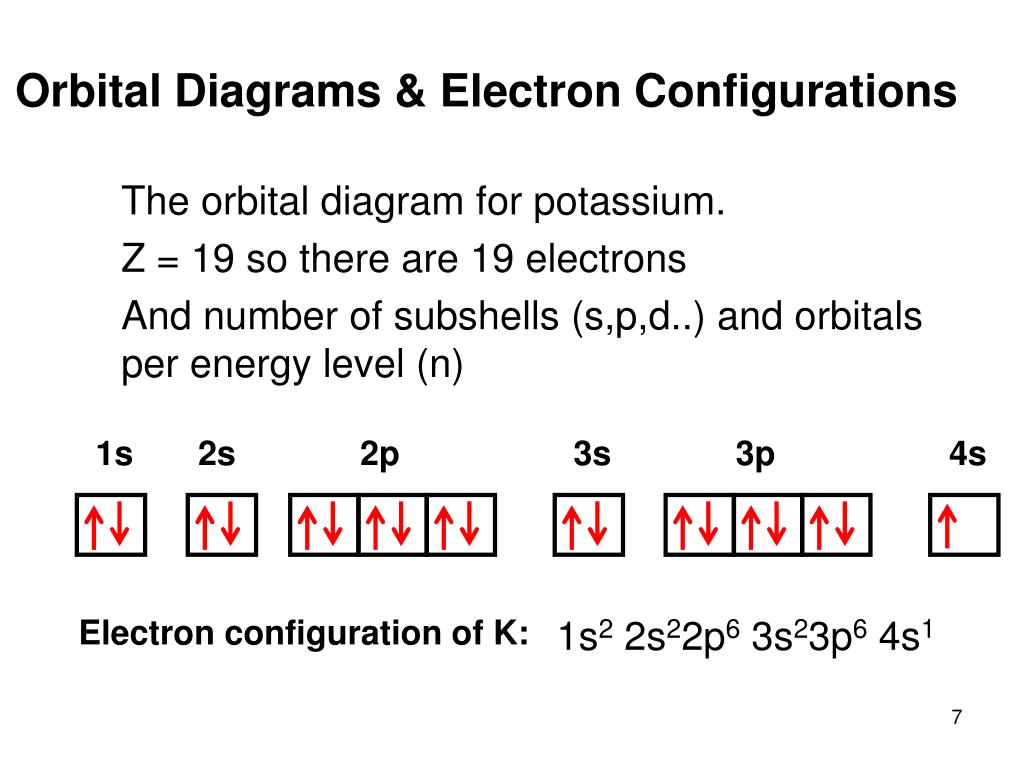
5.3 Vortec Vacuum Diagram. 5 3 vortec engine diagram circuit diagram maker 5 3 vortec engine diagram as well as 2000 chevy 5 3 engine intake diagram further gm 3100 v6 engine diagram in addition were is the vacuum line for 5 3 liter vortec engine diagram imageresizertool 5 3 liter vortec engine diagram also oxygen sensor for chevy truck further chevrolet cavalier 2 0 2000 specs and images in. SO3 Molecular Geometry, Lewis Structure, and Polarity Explained. SO3 stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes. Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. What is the electron dot configuration for sodium? The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining electron in the 3s. Therefore the sodium electron configuration will be 1s22s22p63s1.
By Hund's rule, the electron configuration of carbon, which is 1s2 2s2 2p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Which of the following has maximum number of unpaired electrons fe3+ Fe2+ Co2+ Co3 ...
Another way of finding the hybridisation of a given molecule is with the help of lone pairs and valence electrons. The number of lone pairs in this molecule is 3, and the number of atoms sharing valence electrons is 2. Hence, 3+2=5 which also determines sp3d hybridisation. The shape of I3- Ion. The shape of the molecule I3- is Linear.
A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao molecular orbital method in particular.
The p orbital can hold up to six electrons. The atomic number of sodium is 11. And like it was said before. The Electron Configuration of Sodium. 1s 2 2s 1 He2s 1. This video shows how to draw the orbital diagram of Sodium Na. Favorite Answer Sodium 1s2 2s2 2p6 3s1 Iron 1s2 2s2 2p6 3s2 3p6 3d6 4s2 I cant give colbalt as it doesnt exist.
Carbonate mineralization is reasonably well-understood in the Ca-CO2-H2O system but continuously poses difficulties to grasp when Mg is present. One of the outstanding questions is the lack of success in dolomite MgCa(CO3)2 crystallization at atmospheric conditions. The conventional view holds that hydration retards the reactivity of Mg2+ and is supported by solvation shell chemistry.
ClO3- Lewis Structure, Molecular Geometry, Hybridization & Shape. May 3, 2021. Posted by Priyanka. 17 Apr. The chemical formula ClO 3- represents Chlorate ion. Chlorine can reach oxidation states of +1, +3, +5 and +7. In this case, as seen in the figure, Chlorates exist in a +5 oxidation state. With an abundance of oxidizing elements, the ...
Now carbon monoxides mo diagram is. Jmol models of wavefunctions calculated at the rhf321g level. A molecular orbital diagram or mo diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals lcao molecular orbital method in particular.

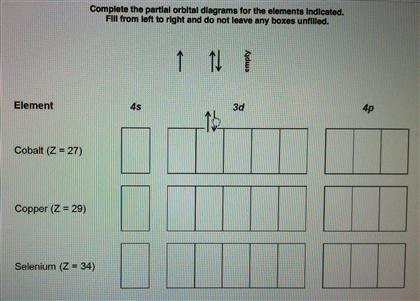
Electron Configuration For Co Co2 And Co3 Cobalt And Cobalt Ions Electron Configuration Electrons Chemical Bond
CHEM 103 Exam 1-6 Final - Complete Solutions, Graded A Exam 1-6 Question 1 1. Convert 1005.3 to exponential form and explain your answer. 2. Convert 4.87 x 10-6 to ordinary form and explain your answer. Question 2 Using the following information, do the conversions shown below, showing all work: 1 ft = 12 inches 1 pound = 16 oz 1 gallon = 4 quarts 1 mile = 5280 feet 1 ton = 2000 pounds 1 quart ...
The physical realization of Chern insulators is of fundamental and practical interest, as they are predicted to host the quantum anomalous Hall (QAH) effect and topologically protected chiral edge ...

Solved 10 What Is Coulomb Law What Happens To Potential Energy When Two Particles Of The Same Charge For Example Two Electrons Are Moved Closer To One Another 11 Explain Why The Radius
What is Fe2 and Fe3. So the electron configuration of Fe can be given as follows. Iron is widely used as a structural material due to its high strength and durability. And the electronic configuration of Fe³ is. The electron configuration for Fe2 will be 1s2 2s2 2p6 3s2 3p6 4s2 3d4 because it has lost two electrons.
NO3 Lewis Structure, Molecular Geometry, and Hybridization. NO3 is a polyatomic ion with a negative charge. So, it is also referred to by the name of nitrogen oxoanion. The compound has its chemical name as nitrate formed after nitric acid looses a proton from it. Nitrate is an important source of nitrogen and oxygen.
The electron in its 1s orbital is shared with the Carbon atom. The carbon atom, which is also the central atom, is the one that requires hybridized orbitals to accommodate the shared electrons. We can find out the hybridization of the atom by using some simple and quick methods such as Steric Number and Number of sigma bonds.
A gaseous Co3+ ion in the ground state has four unpaired electrons. In the same manner how many unpaired electrons are in an atom of carbon in its ground state? By Hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2 , is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ...
CO32- Molecular Orbital (MO) Diagram What is MO theory? Molecular Orbital Theory is a concept of quantum mechanics that is used to decipher the chemical bonding nature inside different molecular structures. This is a complex yet useful tool that helps in sketching MO diagrams for better understanding.

Solved 3 Arrange The Following Elements In Order Of Decreasing Atomic Radius Cs Sb S Pb Se 4 Write The Electronic Configurations For Each Of The Following Ions A 02 B Br C












0 Response to "40 co3+ orbital diagram"
Post a Comment