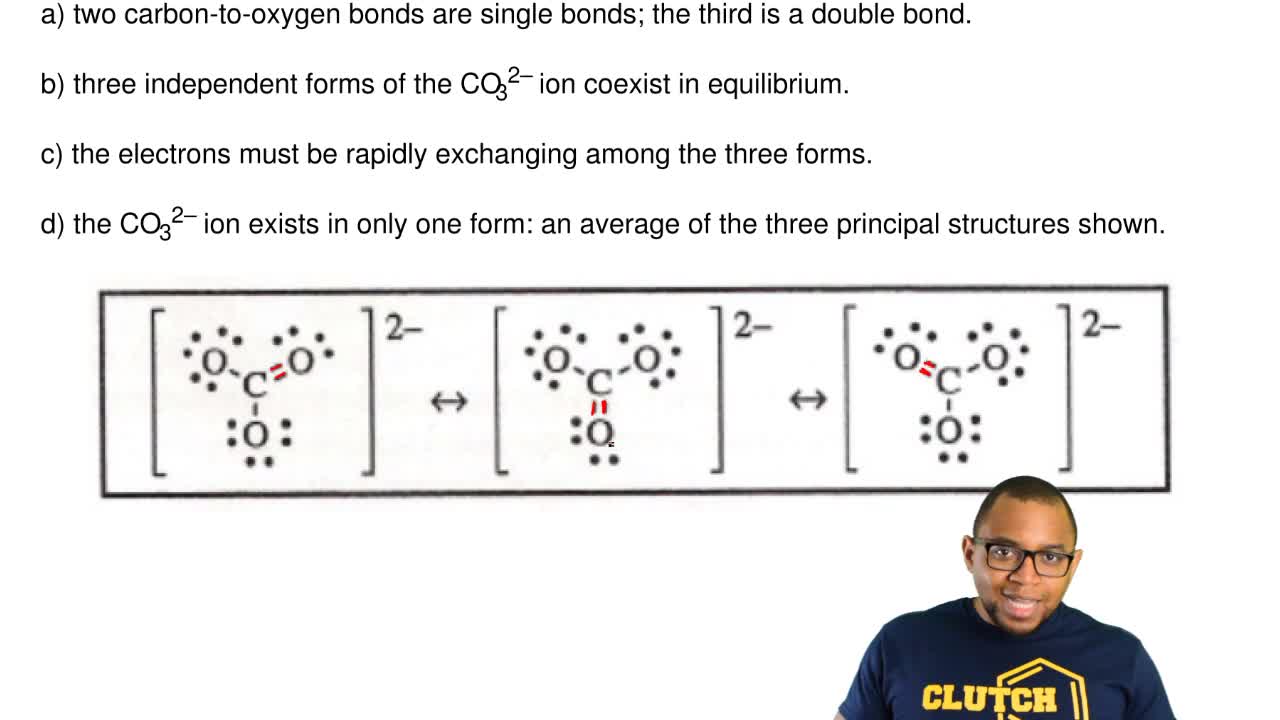
40 lewis diagram for co3-2
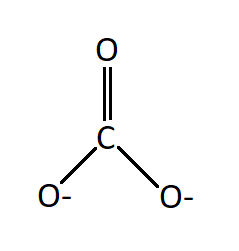
However, the carbonate anion, CO3^2- does have a Lewis dot structure. What is Lewis dot structure for CaCO3? CaCO3 does not have a lewis structure because this molecule is composed of ions. What is the Lewis structure of CO3 2-. Answer (1 of 7): CO3 2- is carbonate.a carbonate is a salt of carbonic acid (H2CO3),characterized by the presence of the carbonate ion, a polyatomic ion with the formula of CO3 2-. The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C (=O) (O–)2.
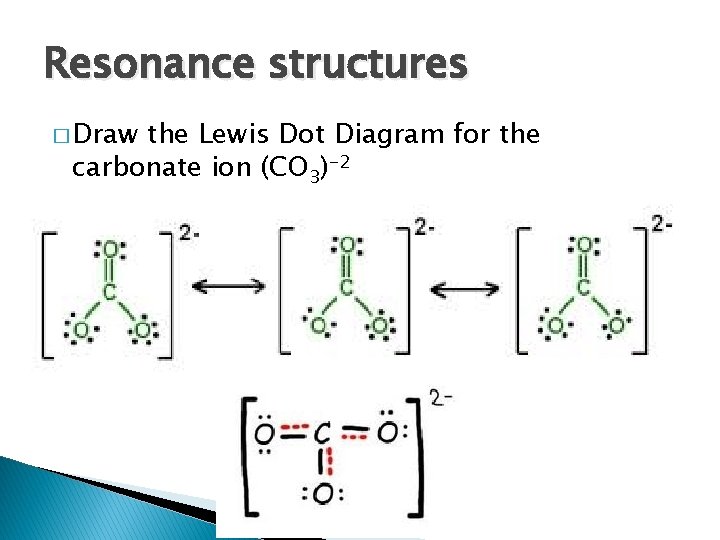
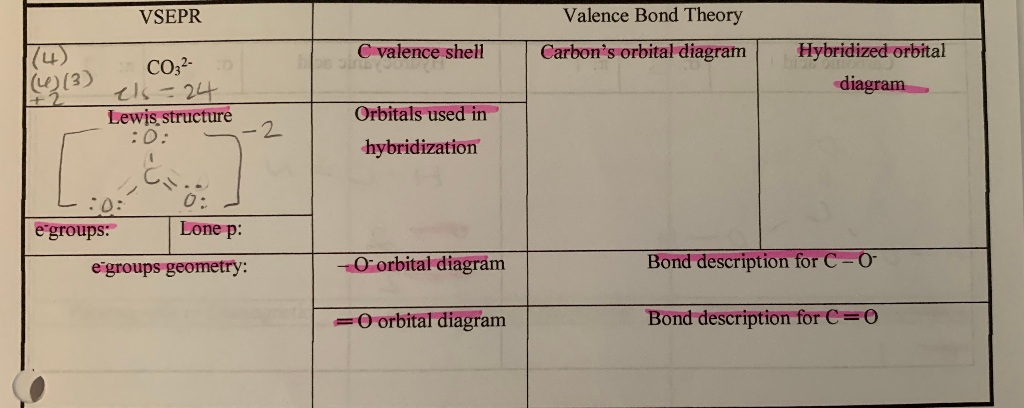
Lets learn step-by-step how to draw Lewis dot structure CO3 -2. Learn how to draw Lewis structures for ions carbonate ion (electron dot structure of CO3 -2)....
Lewis diagram for co3-2
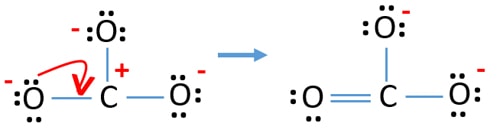
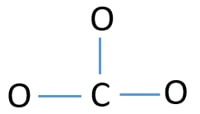
I quickly take you through how to draw the Lewis Structure of CO3 2- (Carbonate Ion). I also go over the resonance, hybridization, shape and bond angle. Apr 04, 2021 · Reply: The form of CO3^2- is trigonal planar. It has the carbon within the center with three oxygens bonded to it. One C-O bond is a double bond, whereas the opposite two C-O bonds are single bonds. The oxygens with the one bonds have three lone pairs every and the oxygen with the double bond has two lone pairs. A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion).For the CO3 2- structure use the periodic table to find the total nu...
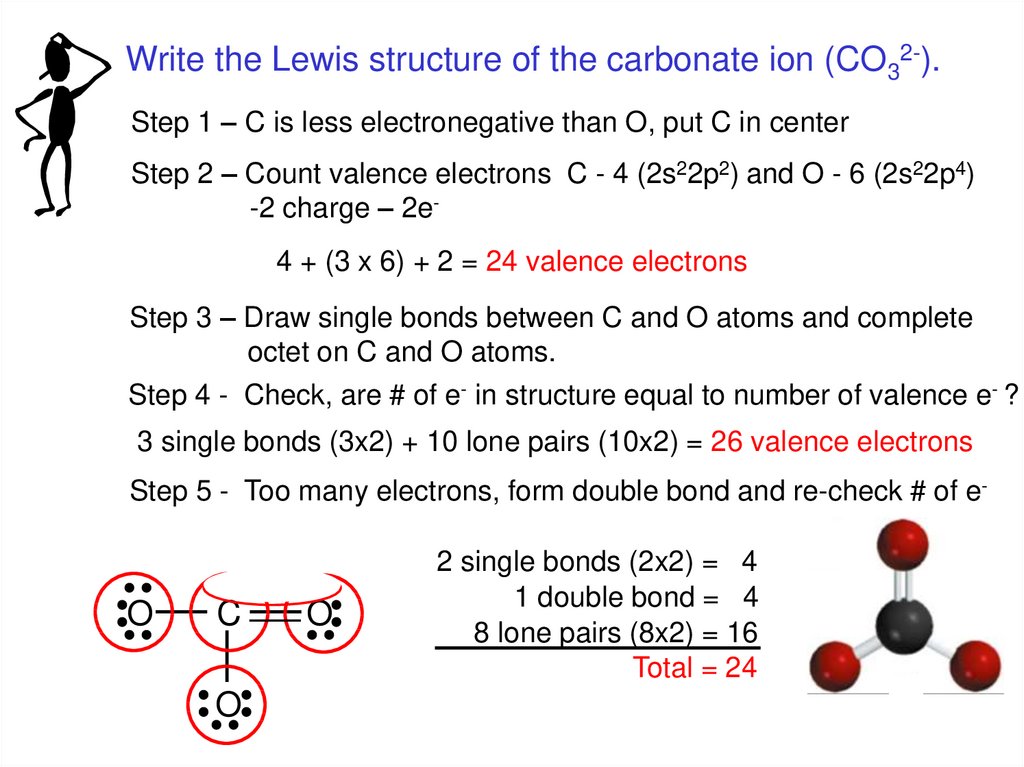
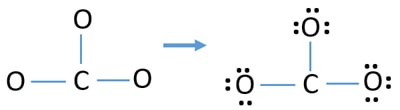
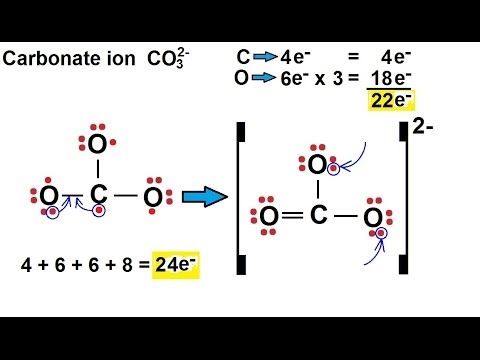
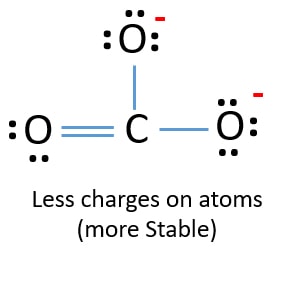
Lewis diagram for co3-2. Lewis Structure for CO 3 2-| Carbonate ion. Lewis structure of carbonate ion is drawn in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of CO 3 2-.After finishing the lewis structure of CO 3 2-, there should be a -2 charge and it should be stabile structure. If you are reading this article, you have probably already come across this term, haven’t you? It is needless to mention when one learns about the nature of chemical bonding across atoms and molecules, Lewis Structure is a concept that we cannot simply factor out. To get a quick and clear overview of the atomic bonding across elements, all we need to do is to first sketch a 2D diagrammatic representation of the given molecule. Lewis Structure is the name given to such a skeletal diagram where we use the symbols of the atoms and use dots to represent the valence shell electrons. Hence, Lewis Structure is also commonly called Electron Dot Structure. Let us proceed to draw the most appropriate LS diagram of CO32- ion. Step 1: Count the Total Number of Valence Electrons. In CO32- ion, we have one carbon atom and three oxygen atoms along with two negatively charged electrons carrying the charge. Valence electrons refer to the number of electrons in the outermost shell of an atom around t... CO3 2- Lewis Structure. CO3 2- lewis structure, first, we calculate Q. Q = Valance electron of all-atom + no of -ve charge – no of +ve charge . Q = (22 + 2 – 0) Q = 24. B.P e – = 2 × no of bonds. B.P e – = 2 × 3. B.P e – = 6 e – L.P e – = Q – B.P e – L.P e – = 24 – 6. L.P e – = 18. We complete the octet of corner atoms ... Draw the Lewis structure of the carbonate ion, CO 3 2−. (Assign lone pairs, radical electrons, and atomic charges where appropriate.) Calculate the electrons required (ER), valence electrons (VE), and shared pairs (SP).
This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. This video discusses the resonance structu... A simple notation used to represent valence electrons in an atom is called Lewis symbol. According to him, atoms achieve stable octet by gaining, loosing or ... A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion).For the CO3 2- structure use the periodic table to find the total nu... Apr 04, 2021 · Reply: The form of CO3^2- is trigonal planar. It has the carbon within the center with three oxygens bonded to it. One C-O bond is a double bond, whereas the opposite two C-O bonds are single bonds. The oxygens with the one bonds have three lone pairs every and the oxygen with the double bond has two lone pairs.
I quickly take you through how to draw the Lewis Structure of CO3 2- (Carbonate Ion). I also go over the resonance, hybridization, shape and bond angle.






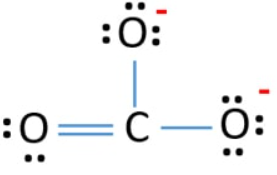





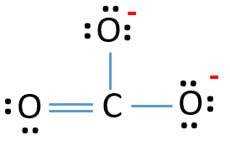

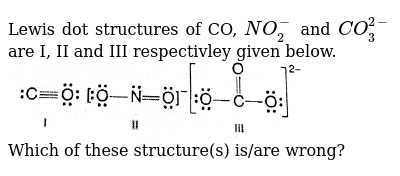








0 Response to "40 lewis diagram for co3-2"
Post a Comment