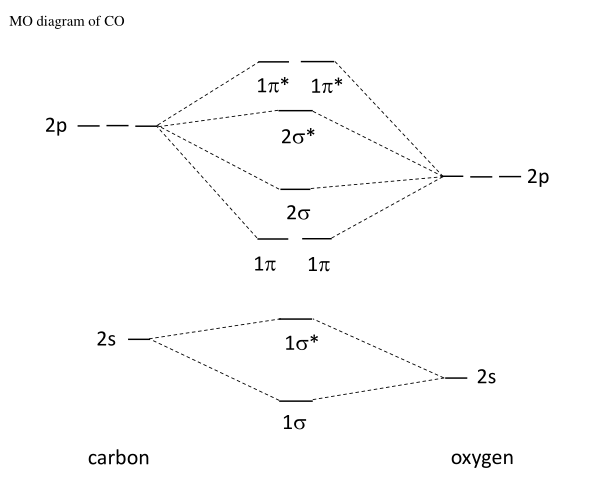
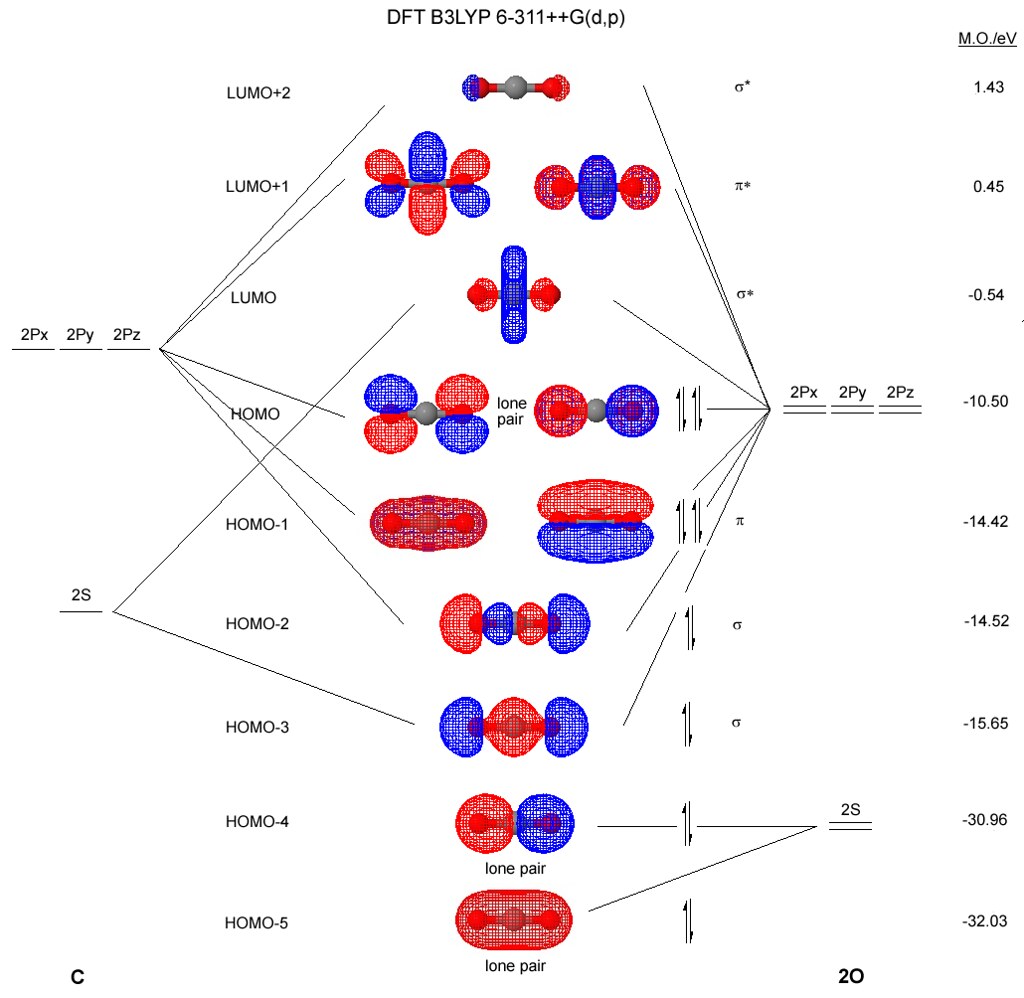
43 carbon molecular orbital diagram
Feb 28, 2017 · This gives us a 2-carbon pi orbital in the centre flanked by two one-carbon orbitals on the sides. 7. The Full Molecular Orbital Diagram For The Butadienyl System (n=4) Now that we have all the pieces, all we need to do to construct the molecular orbital diagram for the butadienyl system is to arrange the orbitals in order of increasing energy. Carbon dioxide — Carbon dioxide's molecular orbitals are made by the linear combination of atomic orbitals of the same irreducible representation that ...
Download scientific diagram | Qualitative molecular orbital diagram for MoC. The 10 orbital is mainly a carbon 2 s orbital. The 11 and 5 orbitals ...

Carbon molecular orbital diagram
Carbon nanotubes (CNTs) ... which may range from 0 (inclusive) to 60 degrees clockwise (exclusive). If the diagram is drawn with u horizontal, the latter is the tilt of the strip away from the vertical. Here are some unrolled nanotube diagrams: Chiral nanotube of the (3,1) type. Chiral nanotube of the (1,3) type, mirror image of the (3,1) type. Nanotube of the (2,2) type, the narrowest ... Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds. 2 Dec 2016 — The problem provides you with the MO diagram for the C2 molecule, ... As you know, a neutral carbon atom has a total of 6 electrons.1 answer · Here's what I got. Explanation: The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add an electron ...
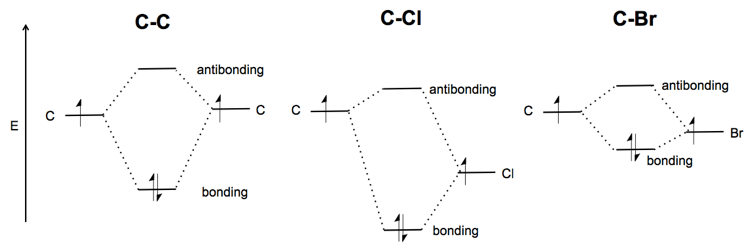
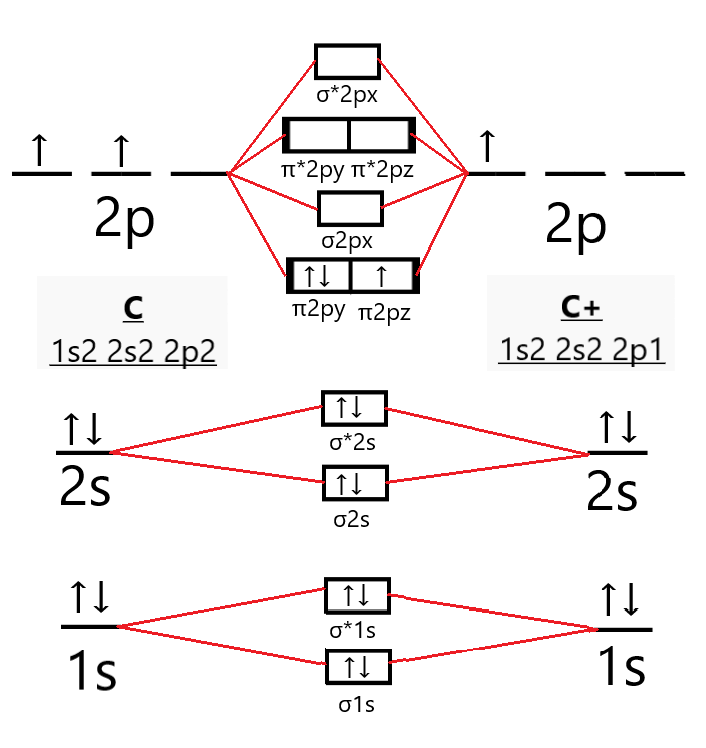
Carbon molecular orbital diagram. 26 Feb 2018 — write molecular orbital configuration of c2+ predict magnetic behaviour and calculate its bond order. ... The C2 molecule is diamagnetic because ... molecular orbitals in the diagram suggest a double bond. c. The ... 5.11 The molecular orbital description of KrF+ would predict that this ion, which has the same number of valence electrons as F2, would have a single bond. KrF2 would also be expected, on the basis of the VSEPR approach, to have single Kr–F bonds, in addition to three lone pairs on Kr. Reported Kr–F distances: KrF+: 176.5 ... 22 Dec 2020 · 1 answerDraw and explain the molecular orbital diagram of carbon molecule. · 1. Electronic configuration of C atom – 1s2 2s2 2p · 2. Electronic ... The next element has two electrons and the second electron fills the 1s orbital because there are only two possible values for the spin quantum number used to distinguish between the electrons in an orbital. He (Z = 2): 1s 2. The third electron goes into the next orbital in the energy diagram, the 2s orbital. Li (Z = 3): 1s 2 2s 1
7: diagram showing how the electrons fill based on the Aufbau principle. The π bonding orbital is lower in energy than the nonbonding p orbital. Since every ... example, neutral carbon has 6 electrons. The electronic configuration for thiswould be 1s22s22p2, meaningthere are2electronsin subshell1s, 2electronsin subshell2s, and2electronsin subshell 2p. To figure out the configuration on your own, you can follow the orbital diagram to … May 09, 2018 · The electronic configuration of carbon and oxygen atom are 1s²2s²2p² and 1s²2s²2p⁴ respectively. There are 4 electrons in the outer shell of carbon and 6.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear ... In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital. The bonding MO is occupied by two electrons of opposite spin, the result being a covalent bond. 19 May 2019 — This angle suggests that the carbon atoms are sp2 hybridized, ... IFigure 10.7.2: Molecular Orbital Energy-Level Diagram for π Bonding in ... 07.11.2021 · OF2 Molecular Geometry. We have already found the 2D Lewis Structure diagram of the Oxygen Difluoride molecule. Now, we are going to decipher the 3D molecular shape. Via Lewis Structure, we have realized the type of bond formed and the number of lone or unbonded pairs of valence electrons present in an OF2 molecule. Nov 02, 2015 · If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion. It's not ...

Draw The Molecular Orbital Diagram For O 2 What Is Its Bond Order State Whether This Molecule Is Paramagnetic Or Diamagnetic From Chemistry Coordination Compounds Class 12 Cbse
Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.

Draw The Molecular Orbital Diagram For Indicate The Point Group Symmetry And Draw With Bonding And Homeworklib
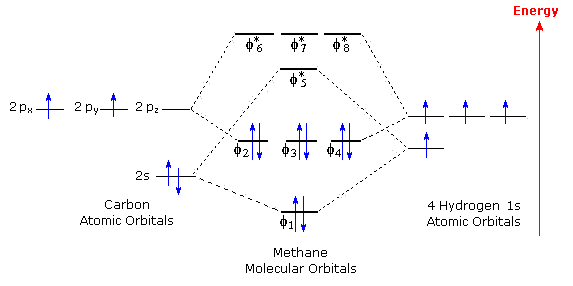
The molecular orbital diagram for methane CH 4 illustrates how valence hydrogen and carbon atomic orbitals combine to form an equal number of molecular orbitals. Figure 6.7 . The four linear combinations of hydrogen 1s atomic orbitals represented by expressions (6.4) through (6.7) that match the tetrahedral symmetry of the carbon 2s and 2p atomic orbitals in CH 4 .
2 Dec 2016 — The problem provides you with the MO diagram for the C2 molecule, ... As you know, a neutral carbon atom has a total of 6 electrons.1 answer · Here's what I got. Explanation: The problem provides you with the MO diagram for the C2 molecule, so all you really have to do here is add an electron ...
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
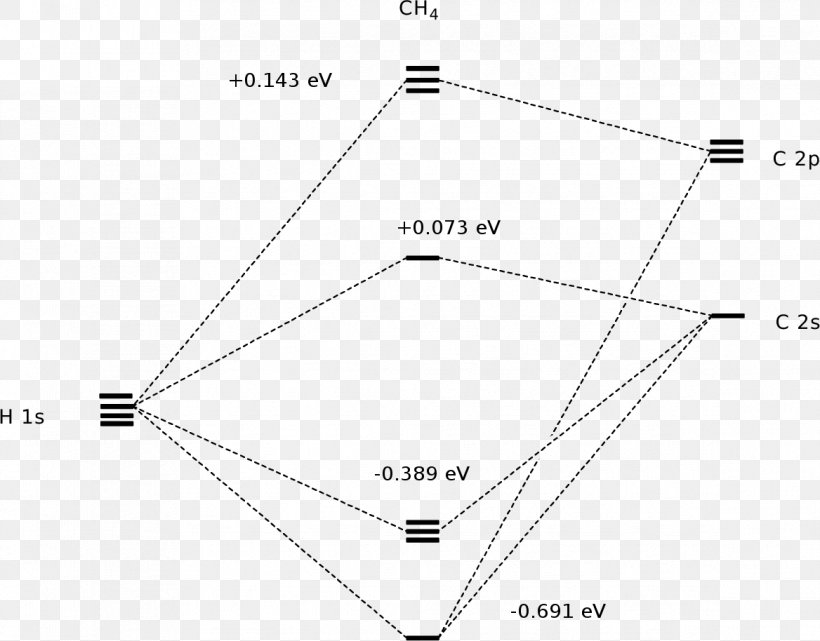
Molecular Orbital Diagram Molecular Orbital Theory Methane Molecule Png 1168x914px Molecular Orbital Diagram Area Atomic Orbital
Carbon nanotubes (CNTs) ... which may range from 0 (inclusive) to 60 degrees clockwise (exclusive). If the diagram is drawn with u horizontal, the latter is the tilt of the strip away from the vertical. Here are some unrolled nanotube diagrams: Chiral nanotube of the (3,1) type. Chiral nanotube of the (1,3) type, mirror image of the (3,1) type. Nanotube of the (2,2) type, the narrowest ...

93 Chemical Bonding 40 Covalent Bonding 39 Molecular Orbital Theory 12 Heteronuclear Diatomic Molecules 3 Madoverchemistry Com
Solved Draw The Mo Diagram For Cs2 Showing All Of The Possible Orbitals For Each Atom Or Group Show Which Interactions Would Be Bonding Non Bond Course Hero

Write The Molecular Electronic Configuration Of F2 And C2 Draw The Energy Level Diagram Calculate Brainly In
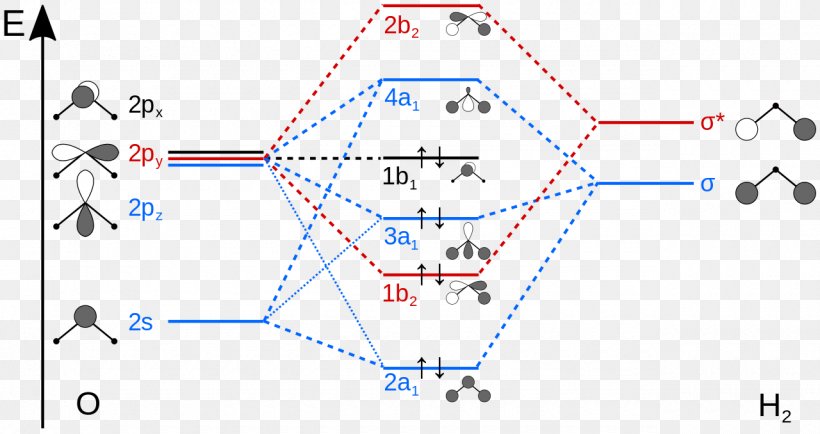
Molecular Orbital Diagram Atomic Orbital Molecule Molecular Orbital Theory Png 1280x678px Molecular Orbital Diagram Area Atom

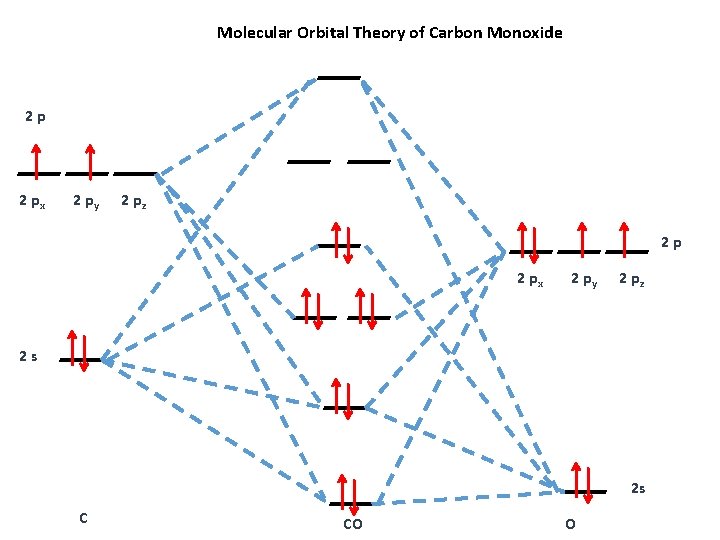
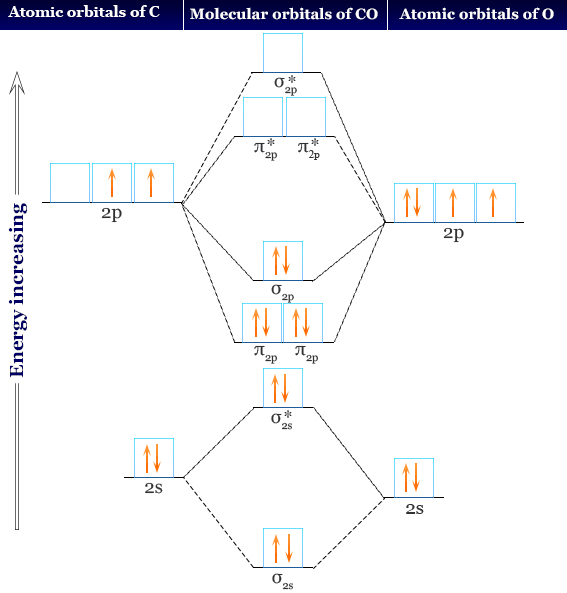
How To Rationalise With Mo Theory That Co Is A Two Electron Donor Through Carbon Chemistry Stack Exchange



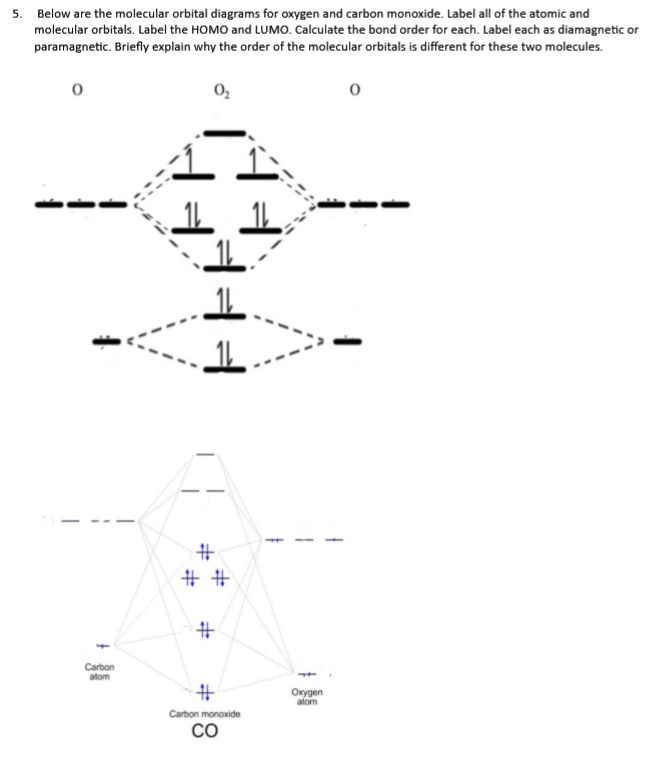

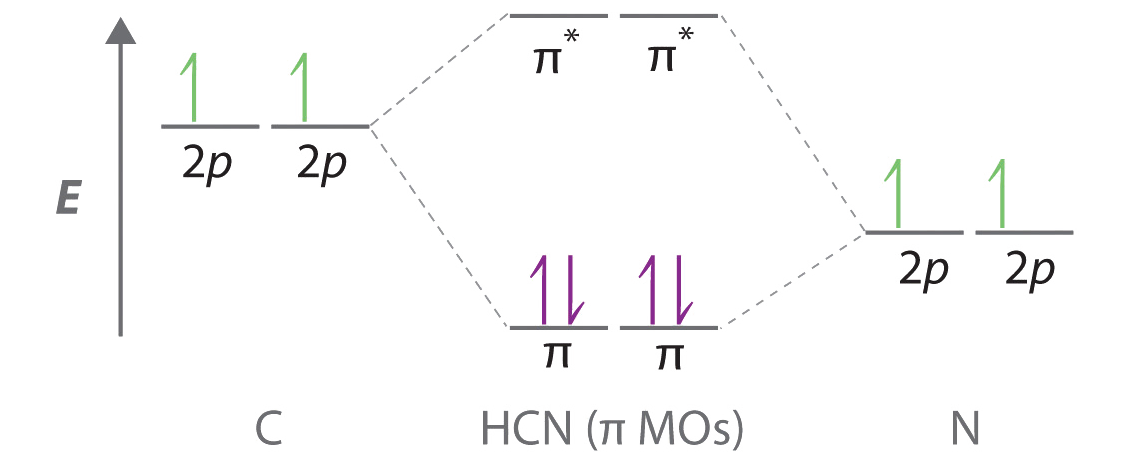









0 Response to "43 carbon molecular orbital diagram"
Post a Comment