43 cr3+ orbital diagram
Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ... Answer to: 1. Write orbital diagrams for each of these ions. *a. V5+ *b. Cr3+ *c. Ni2+ *d. Fe3+ 2. Determine if the ion is diamagnetic or...1 answer · Top answer: 1. The orbital diagrams are shown in the figures below. 2. V5+V5+ has all the electrons paired up so it is...
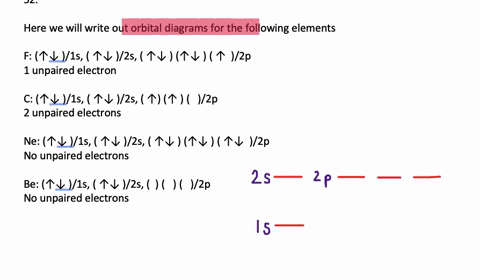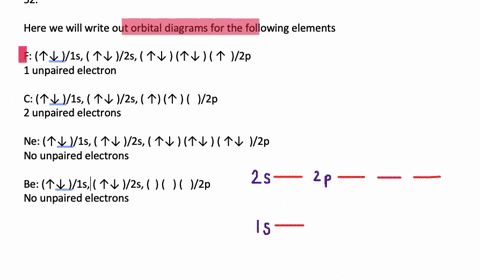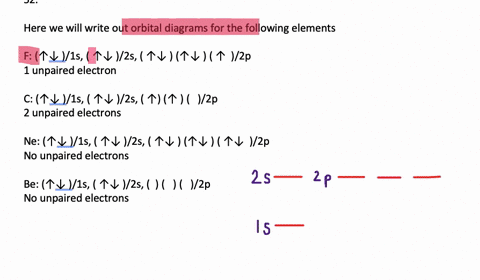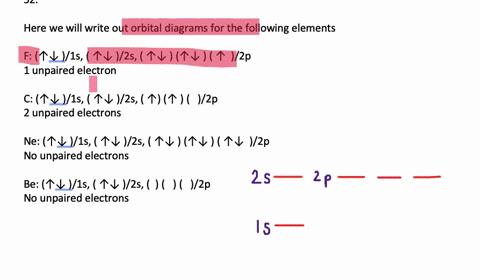
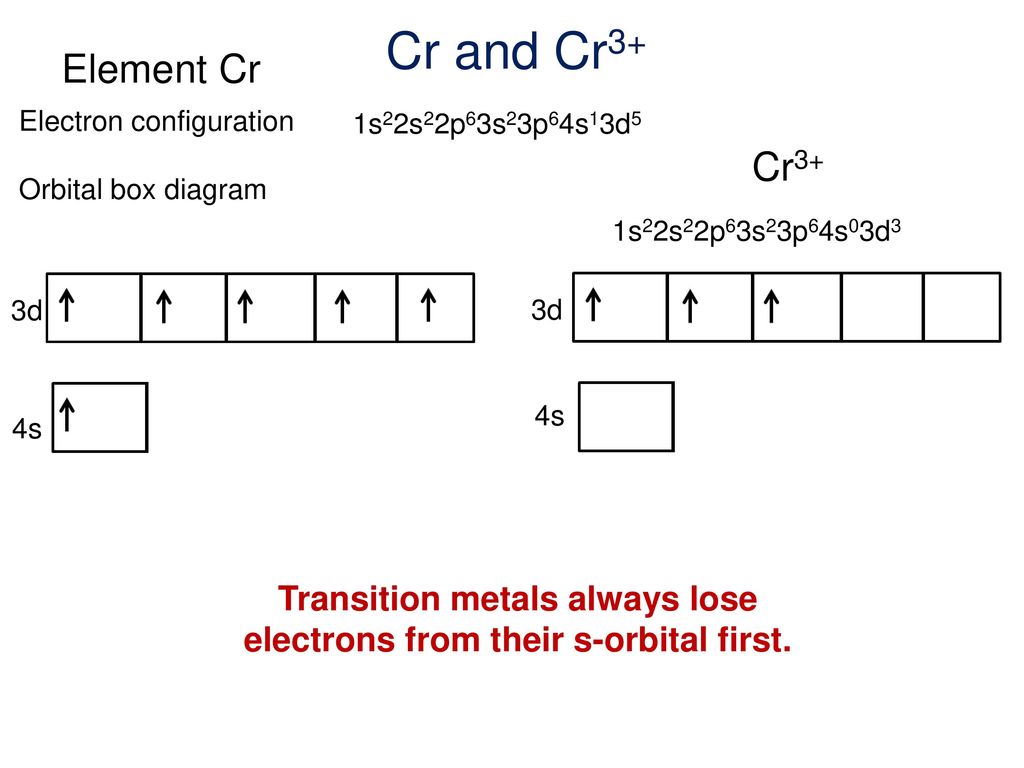
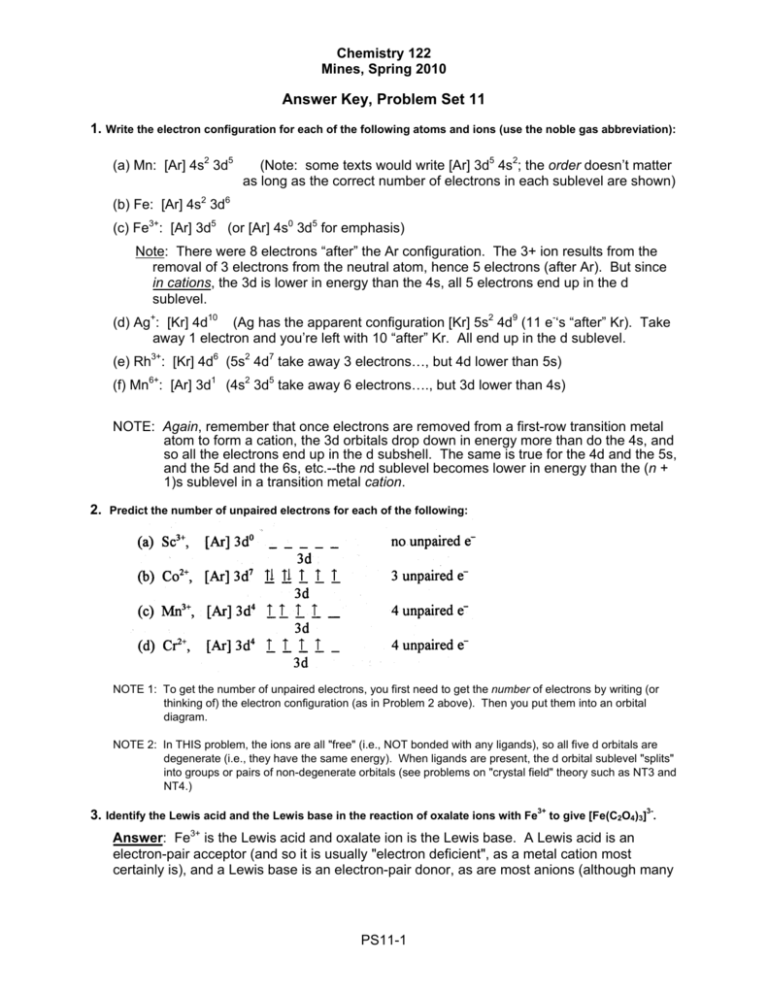
Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3.

Cr3+ orbital diagram
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. V5+ orbital diagram keyword after analyzing the system ... The electronic configuration of Cr having atomic number of 24 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3. Enter the orbital diagram for the ion mo 3. Used in electronics jewelry and coins. Add them in order of increasing orbital energy. Click within the orbital to add electrons. Click within the orbital to add electrons. Write orbital diagram for au. Use the buttons at the top of the tool to add orbitals.
Cr3+ orbital diagram. The electronic configuration of Cr(24) atom is: 1s22s22p63s23p64s13d5 which is half-filled d-orbital. Cr3+ has 3 electrons removed from the outermost shell.19 Nov 2019 Orbital Diagram For Fe3 Examples: Electron Configurations of Transition Metal Ions hen d-block elements form ions, the 4 s electrons are lost first: Ion Electron Configuration Cr 1s22s22p63s23p6 4s13d 5 Cr3+ 1s22s22p63s23p6 3d3 Ion Electron Configuration Fe 1s22s22p63s23p6 4s23d 6 Fe3+ 1s22s22p63s23p6 3d5 Lost 3 electrons - one from 4 s and two ... Electrons are added to a subshell with the same value of the spin quantum number until each orbital in the subshell has at least one electron. Octahedral transition-metal ions with d 1, d 2, or d 3 configurations can therefore be described by the following diagrams. When we try to add a fourth electron, we are faced with a problem. See the answer See the answer done loading. Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+. Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+. Expert Answer.
To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). We first need to find the number... Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In Br or Ar S or Sn Si or Cl. In Br Sn Si. Choose the ... Electron Orbitals And Valence Diagram Electron Configuration Electrons Transition Metal. Electron Configurations For The Third And Fourth Periods Video Khan Academy. 1 Write Orbital Diagrams For Each Of These Ions A V5 B Cr3 C Ni2 D Fe3 2 Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2. The s orbital holds a maximum of 2 electrons. The p orbital can hold 6. The d orbital can hold 10. The f orbital can hold 14 electrons. But, the orbitals overlap. The Madelung rule gives the order: 1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals. can be accommodated in the metal d orbitals. • d0 ions d3 ions - V2+, Ta2+, Cr3+, Mo3+, Mn4+, etc. . σ-ML4 Tetrahedral MO Diagram e. Answer to Write orbital diagram for Mo3+. After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s. Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ Question: Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ This problem has been solved! See the answer See the answer See the answer done loading. Write orbital ... Answer: Go to the following website to find the orbital diagram of any element of any Oxidation state. Orbital Energy Diagram and Atomic Electron Configuration Tool As for an actual diagram (per Wiki) is above. You can also find the electron configuration on both websites. For an overview and ...
Answer (1 of 5): In cr3+ there are three unpaired elctrons in its complex and H2O is slightly weaker ligand and it is not forced the electrons for back donation and pairing so after sp3d2 hybridisation the central metal cr has three unpaired electrons B ut in the case of [ Fe(CN)6]4- the central...

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
The valence electron configuration of Cr is 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1 instead of 1s2, 2s2, 2p6, 3s2, 3p6, 3d4, 4s2 because one of the electron from the s orbital jumped to the d orbital. By distributing its electrons along the empty orbitals, it becomes more stable. Since the ground state for Cr is 1s2, 2s2, 2p6, 3s2, 3p6, 3d5, 4s1.
Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a circle with one slash through it. The electron configuration of Hydrogen is 1(s^1). Source: www ...
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic.
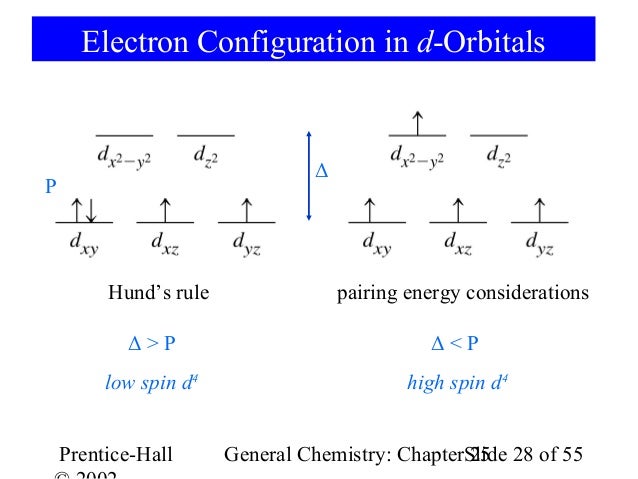
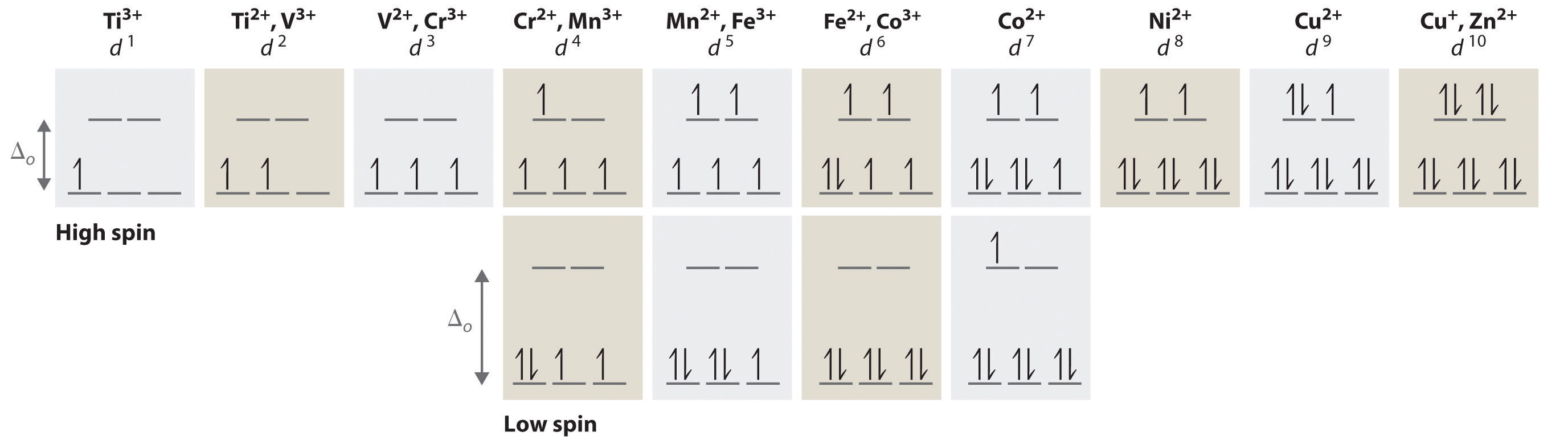
electrons into the same orbital •Πeis a stabilizing energy for electron exchange associated with two degenerate electrons having parallel spin total 3 e 0 c eg* t2g d4HS eg* t2g d8 eg* t2g d6LS total 7 e 3 c total 6 e 3 c LFSE 3 0.4 O 10.6 O 0.6 O LFSE 6 0.4 O 20.6 O
Cr3+ = 3d3. Cr4+ = 3d2. Ni2+ = 3d8 ... from these diagrams. ... quintuplet, can in general be expressed as a function of the 3 orbital angular momentum.5 pages
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
Transcribed image text: Part A Enter an orbital diagram for V5+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all targets will be filled Reset Help P 111 1s 25 38 4s 2p 3p 4p 3d 61 G1 G1 GIG1 G1 GIG1 G1 G1 G1G1G1 G1|| G1 G16161 62 G2 G2 G2 G2 G2 G2 G2 Submit Request Answer Part B Enter an orbital diagram for Cr3 Drag the ...
Problem: Fill in the energy orbital Diagram for the following atom Cr(III). Be sure to label orbitals by n and l quantum numbers, and place electrons in the ...23 Sep 2020
Orbital Diagram: In electron configurations, the electrons in an atom or an ion are assigned to various electronic orbitals. The orbital diagram would show the spin of each electron in the ...
Limitations of MO Diagrams The problem: orbital energy diagrams ignore inter-electron repulsion, i.e., several states comprise the (t 2g)1(e g) 1 configuration,
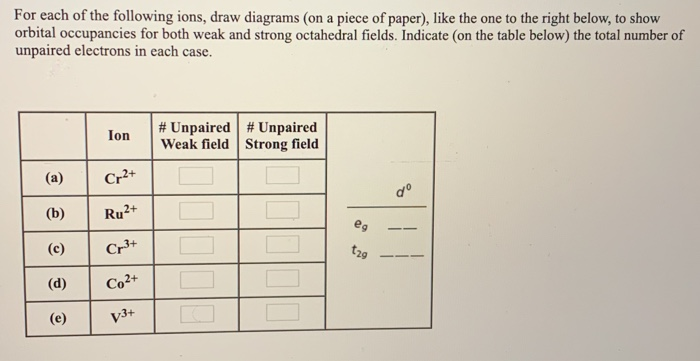
an crystal field splitting diagrams to show orbital occupancies in both weak and strong octahedral fields, and (ii) indicate the number of unpaired electrons in each case. Label . the diagrams (iii) weak or strong field, (iv) high spin or low spin (as appropriate), (v) with the names of the d-orbitals, and (vi) with the appropriate orbital sets ...
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. Therefore the expected electron configuration for Copper will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 . Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s.
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2 D Fe3
To write the configuration for the Manganese ions, first we need to write the electron configuration for just Manganese (Mn). We first need to find the numb...
Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong. Therefore, the ground state electron configuration for Zr 2+ is : [Kr]4d 2 5s 2. In^+1 [Kr] 5s2 4d10. 1s2, 2s2, 2p6. 1) nuclear charge and relative energy of 3d and 4s orbitals, 2) relative e-e repulsions in 3d and 4s orbitals, 3) exchange energy. Booster Classes. Need an editable periodic table to edit? Enter the ...
The electronic configuration of Cr having atomic number of 24 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital.. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3.
orbital type as a superscript, e.g. 1s22p1 would denote an atom with 2 electrons in its 1s orbital, and one in the 2p orbital. The ground state con guration is the lowest-energy con guration. Marc R. Roussel Multielectron atoms September 14, 2018 11/23
Orbital Diagram: In electron configurations, the electrons in an atom or an ion are assigned to various electronic orbitals. The orbital diagram would show the spin of each electron in the ...
Note that it is 4s13d5 and not 4s23d4 because a half filled d orbital is more stable than a partially filled d orbital. However, the chromium ion Cr3+ possesses 24e− −3e− = 21e− due to the loss of 3 of its electrons. Thus, the electron configuration of Cr3+ is: Cr3+:1s22s22p63s23p64s03d3.
Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
Enter the orbital diagram for the ion mo 3. Used in electronics jewelry and coins. Add them in order of increasing orbital energy. Click within the orbital to add electrons. Click within the orbital to add electrons. Write orbital diagram for au. Use the buttons at the top of the tool to add orbitals.
The electronic configuration of Cr having atomic number of 24 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 1 3d 5 which is half-filled d-orbital. Cr3+ has 3 electrons removed from the outermost shell. Therefore, the electronic configuration comes out to be [Ar]3d3.
Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. V5+ orbital diagram keyword after analyzing the system ...
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2 D Fe3

6 Give The Electron Configuration Use Inert Gas Symbol For Core Electrons And Orbital Diagram For Valence Electrons Homeworklib
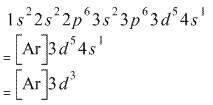
How Is The Electron Configuration Of Cr3 1s2 2s2 2 6 3s2 3p6 3d5 4s1 Ar 3d5 4s1 Ar 3d3 Home Work Help Learn Cbse Forum
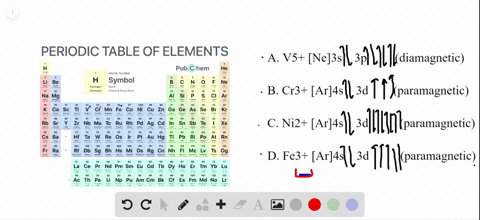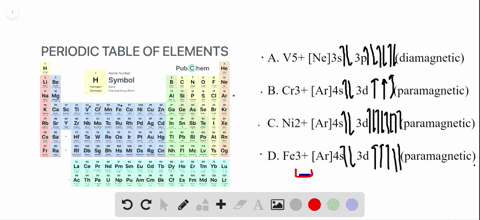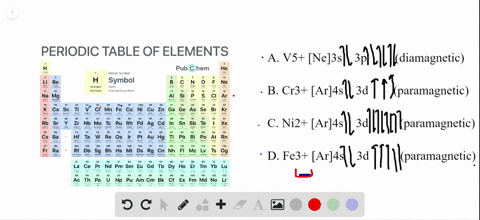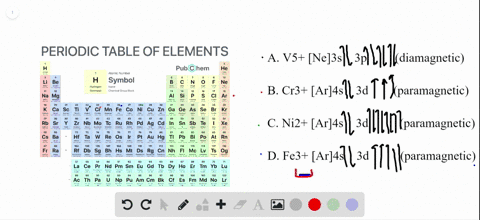
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A V5 B Cr3 C Ni2 D Fe3

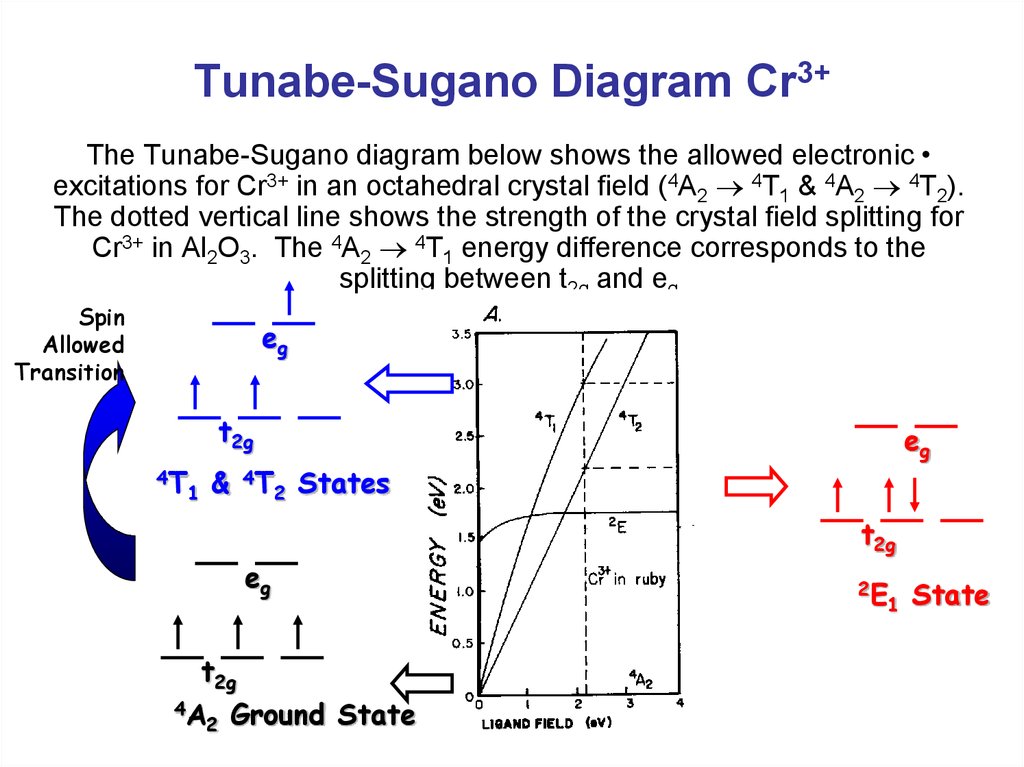

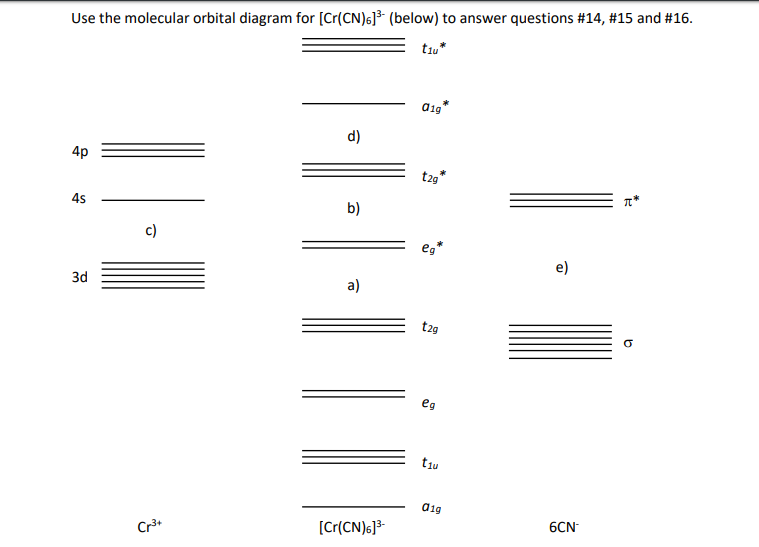
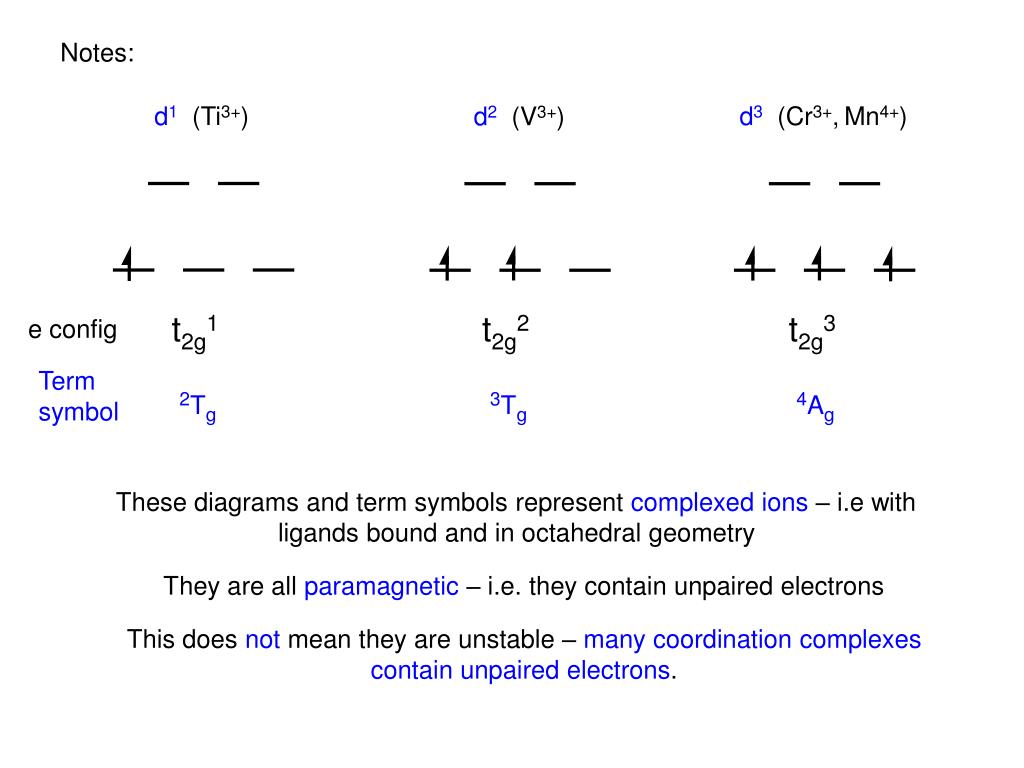

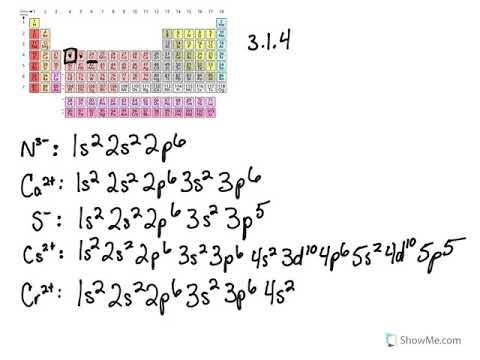










0 Response to "43 cr3+ orbital diagram"
Post a Comment