43 dot diagram for phosphorus
Phosphorus oxychloride is stored and shipped in 3.8-L (1-gal) or smaller glass containers and DOT-specification wooden overpacking. Bulk POCl3 shipments are in nickel -clad tank cars of 15,000-30,000 L (4000-8000 gal) each. Draw the Lewis dot structure for each atom of the molecule to show how many For example, the calcium atom in calcium chloride, CaCl2. The material on this site can not be reproduced, distributed, transmitted, cached or otherwise used, except with prior written permission of Multiply. Draw a Lewis electron-dot structure for an atom of phosphorus.
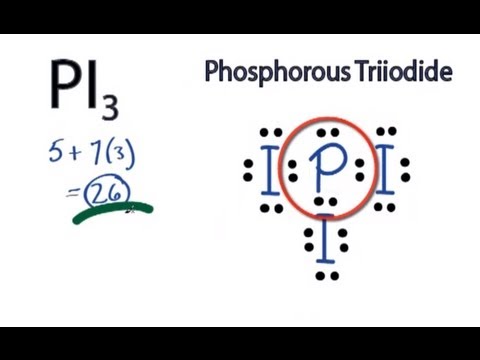
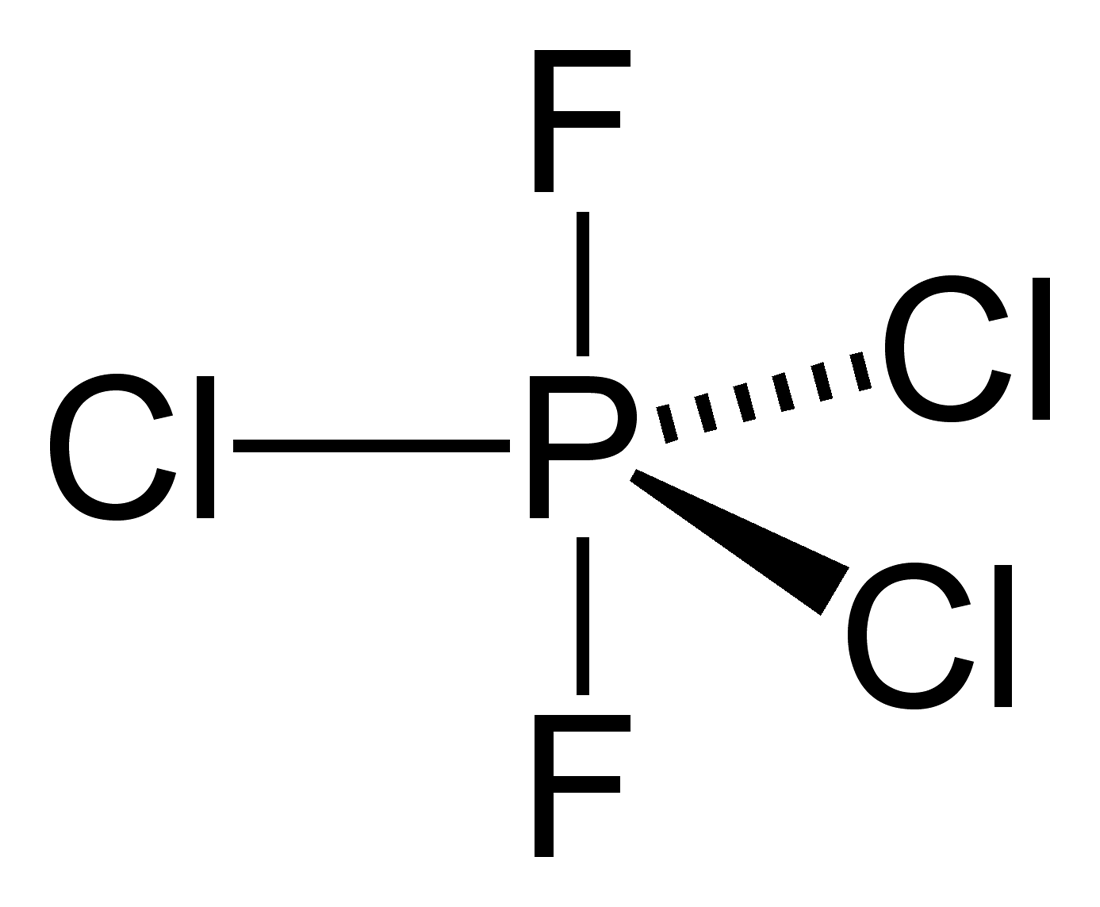
Phosphorus trifluoride (PF3) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle. Phosphorus trifluoride appears as a colorless gas and it is highly toxic in nature similar to carbon monoxide or comparable to phosgene. It is odorless, nucleophile, and weak base in nature and has a chemical formula of PF3.
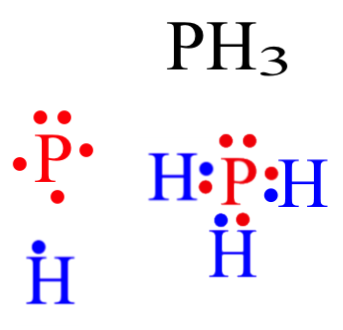
Dot diagram for phosphorus
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds. Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na + ion, we note that the Na atom has a single valence electron in its Lewis diagram ... Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get ...
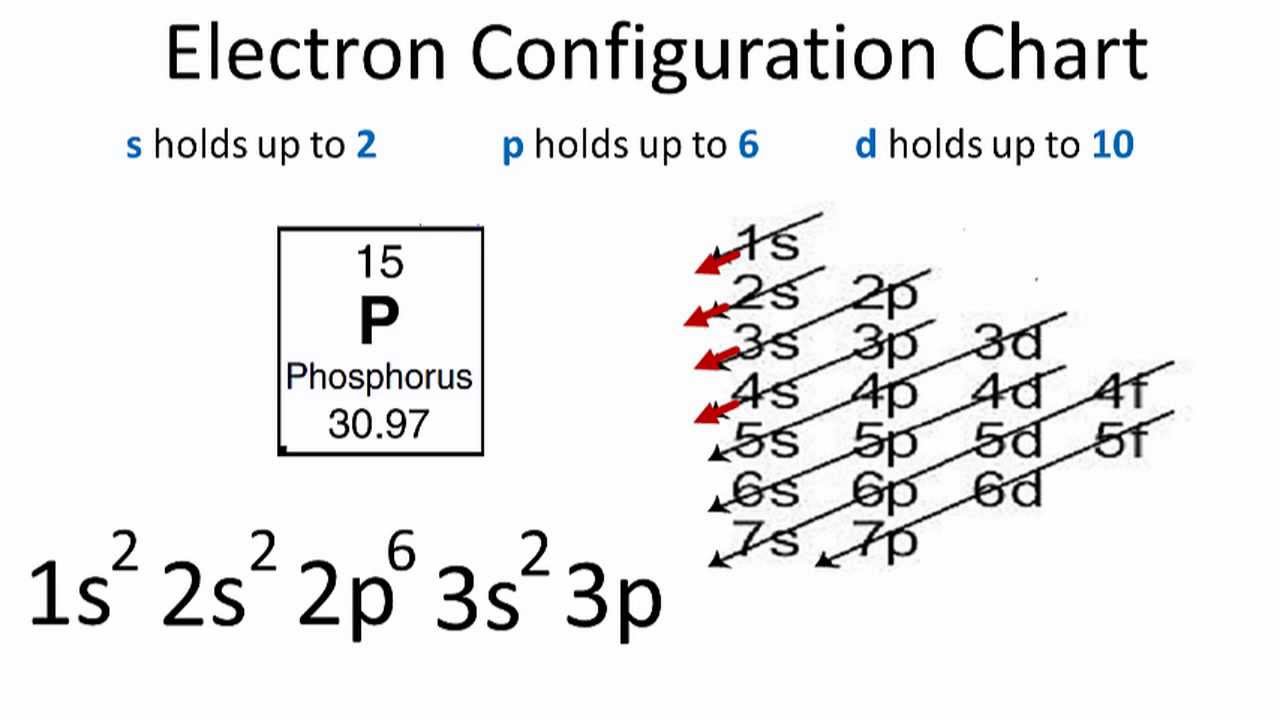
Dot diagram for phosphorus. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 3. Video: Phosphorus Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will ... Phosphorus Dot Diagram. lewis dot diagram for phosphorus answers umm lets see here the lewis dot diagram for phosphorus trifluoride would consist of deep depth of concentration dilemma between the two variables using lewis electron dot diagrams lardbucket what is the lewis electron dot diagram for each element phosphorus argon lewis electron dot diagrams for ions have less for cations or more Lewis diagrams, also called electron-dot diagrams, are used to . Example: Draw the Lewis structure for phosphorus pentafluoride, PF5. The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has.U.S. Department of Transportation (DOT ... The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has tetrahedral geometry. 2)Each P has 5 valence e-s and thus in P4 there are 5×4=20 valence e-s.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the tot... Lewis Dot Diagram: Normally, the Lewis dot diagram is utilized for the representation of the electron pairs, which usually consist of lone pair and valence electrons in it. With elemental phosphorus (white phosphorus, P4) as a bonus.Check me out: http://www.chemistnate.com
Department of Transportation (DOT) Identification number and the corresponding Guide number. The DOT ID is also the UN Number">. More information about the DOT ID/UN number and the guide number can be found at the Emergency Response Guidebook. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. Phosphorus Dot Diagram. Collected from the entire web and summarized to include only the most important parts of it. Can be used as content for research and analysis. Home Blog Pro Plans B2B solution Login. Advanced searches left . 3/3. Search only database of 7.4 mil and more summaries ... What is the Lewis dot structure for PCl3? Phosphorus trichloride (PCl3) contains three chlorine atoms and one phosphorus atoms. In PCl3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. Lewis Dot Structure (Electron Dot Structure) A Lewis dot structure is a quick and easy diagram that shows the valence electrons in an element.In a Lewis structure, the nucleus of the element is represented by its symbol. The valence electrons are represented by dots placed around the symbol in pairs.
Examples for Drawing Lewis Dot Structure for Covalent Bonds . Here, we will be using the determined total number of valence electrons per atom and drawing them in the proper places. Reference the "How to Draw a Lewis Dot Structure" for a Step by Step guide. See the following Lewis dot structure diagrams for a few covalent compounds. Example 1.
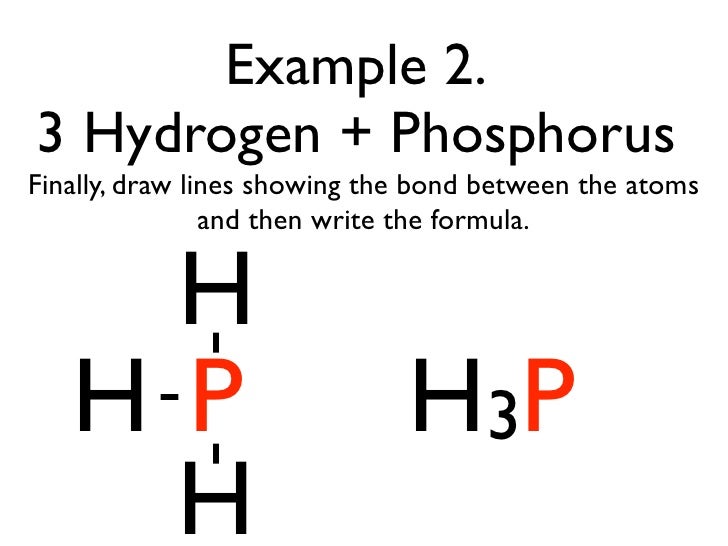
How many dots would be on a Lewis diagram of phosphorus? So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one.
There are three unpaired electrons. As you can see in the electron configuration, the 3p sublevel has room for three more electrons. The orbit filling diagram and the electron dot diagram show the empty spaces for three more electrons and how there are three electrons that aren't paired.
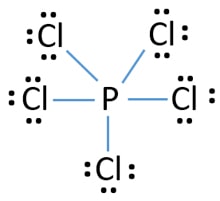
While Chlorine atoms have received the one needed electron, Phosphorus's valency is 3. This could have been a problem, but it can hold the 5 Chlorine atoms, due to its empty 3d orbital. Step 5: Visualizing the diagram, we come up with a Phosphorus in the center, housed by 5 Chlorine atoms. Molecular Geometry of PCl5
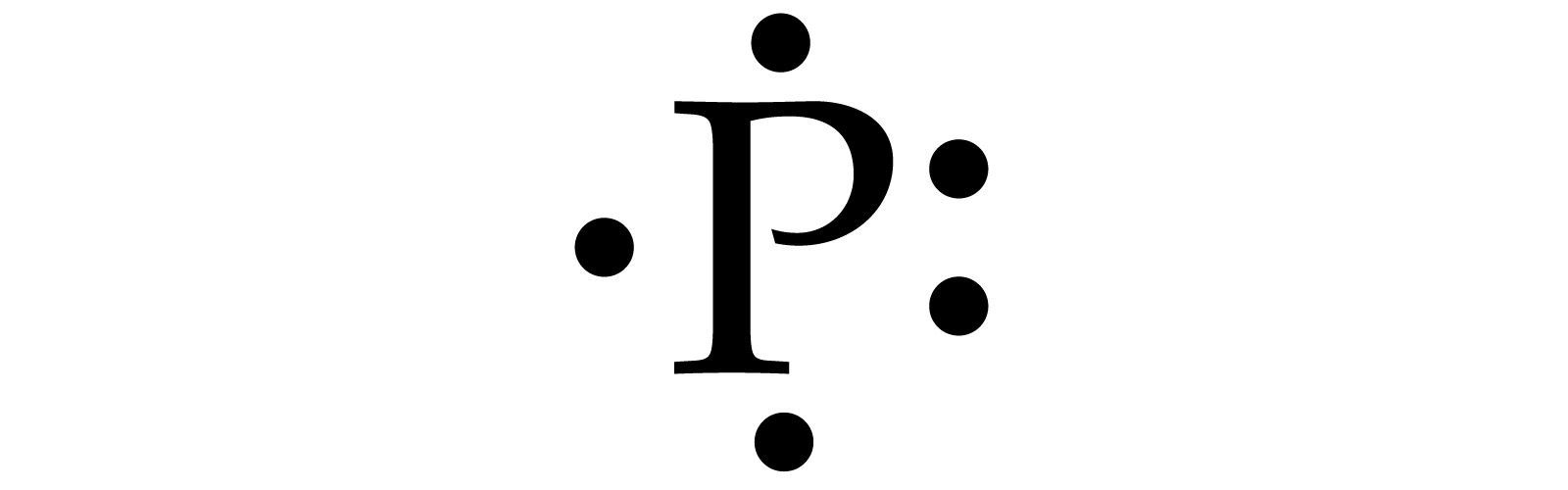
How Would You Draw A Lewis Structure For An Atom That Has The Electron Configuration 1s 2 2s 2 2p 6 3s 2 3p 3 Socratic
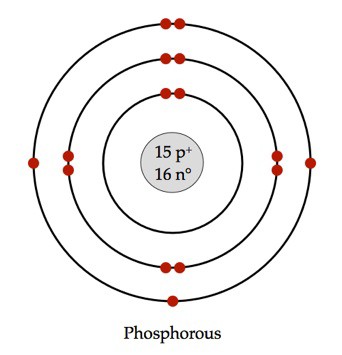
Electron dot diagram of a Phosphorus atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Phosphorus, we got to know, it has 5 valence electrons. So, just represent the 5 valence electrons around the Phosphorus atom as a dot.
So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons. So start with one dot on top, then one dot to the right, one dot on the bottom, one dot to the left, and another dot on top, next to the first one.

The Electron Configuration For Phosphorous Is 1s22s22p63s23p3 What Is The Lewis Electron Dot Diagram Brainly Com
Phosphorus at Chemical schematron.org Basic Information °C ( K, °F) Number of Protons/Electrons: 15 [Bohr Model of Phosphorus], Number of.Bohr diagram for phosphorus furthermore atoms as well as electron configurations in addition plutonium electron diagram further chlorine electron dot diagram also carbon dioxide dot and cross diagram along ...
Key Points To Consider When Drawing The PCl3 Electron Dot Structure. A three-step approach for drawing the PCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the PCl3 molecule, to add valence electrons around the phosphorus atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get ...
Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na + ion, we note that the Na atom has a single valence electron in its Lewis diagram ...
Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Phosphorus Trichloride is widely used in manufacturing Phosphites and other organophosphorus compounds.

Lewis Dot Notes Lewis Dot Diagrams Illustrates The Number Of Valence Electrons Valence Electrons Number Of Electrons In Outer Shell Placed Around Ppt Download

15 P Phosphorus Electron Shell Structure Schoolmykids Phosphorus Element Element Chemistry Electron Affinity
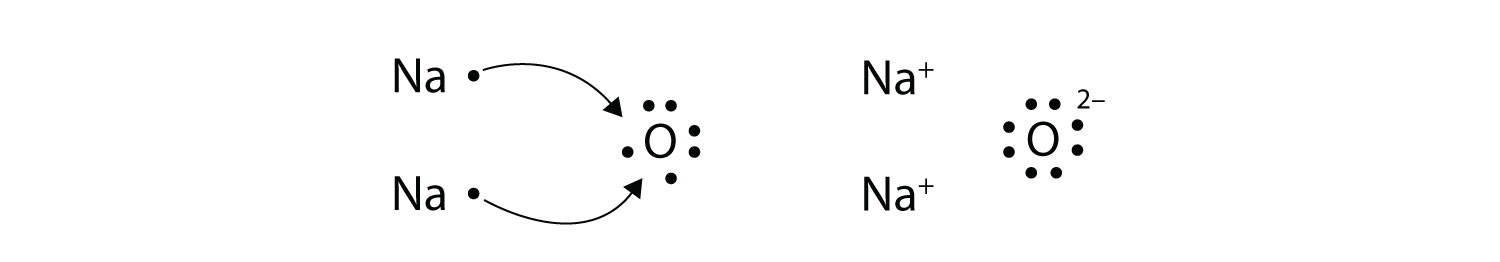
Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library


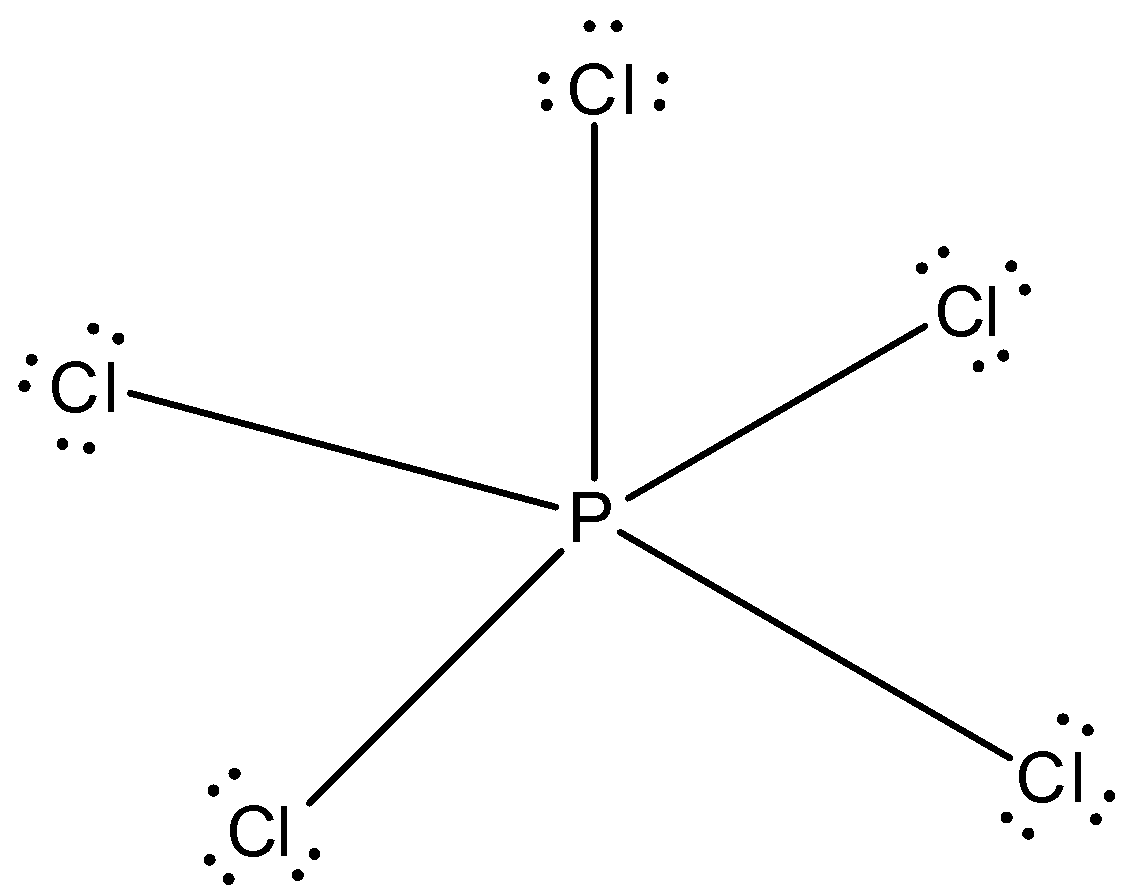












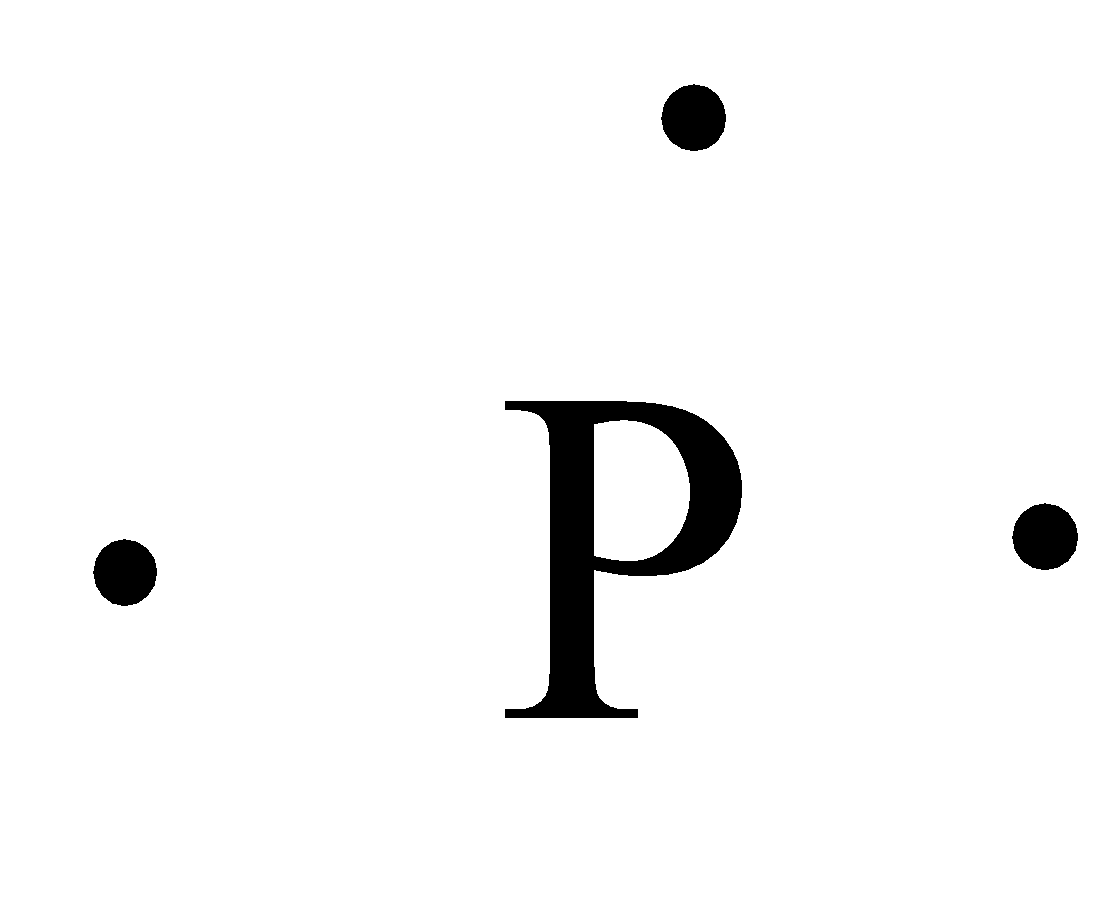






0 Response to "43 dot diagram for phosphorus"
Post a Comment