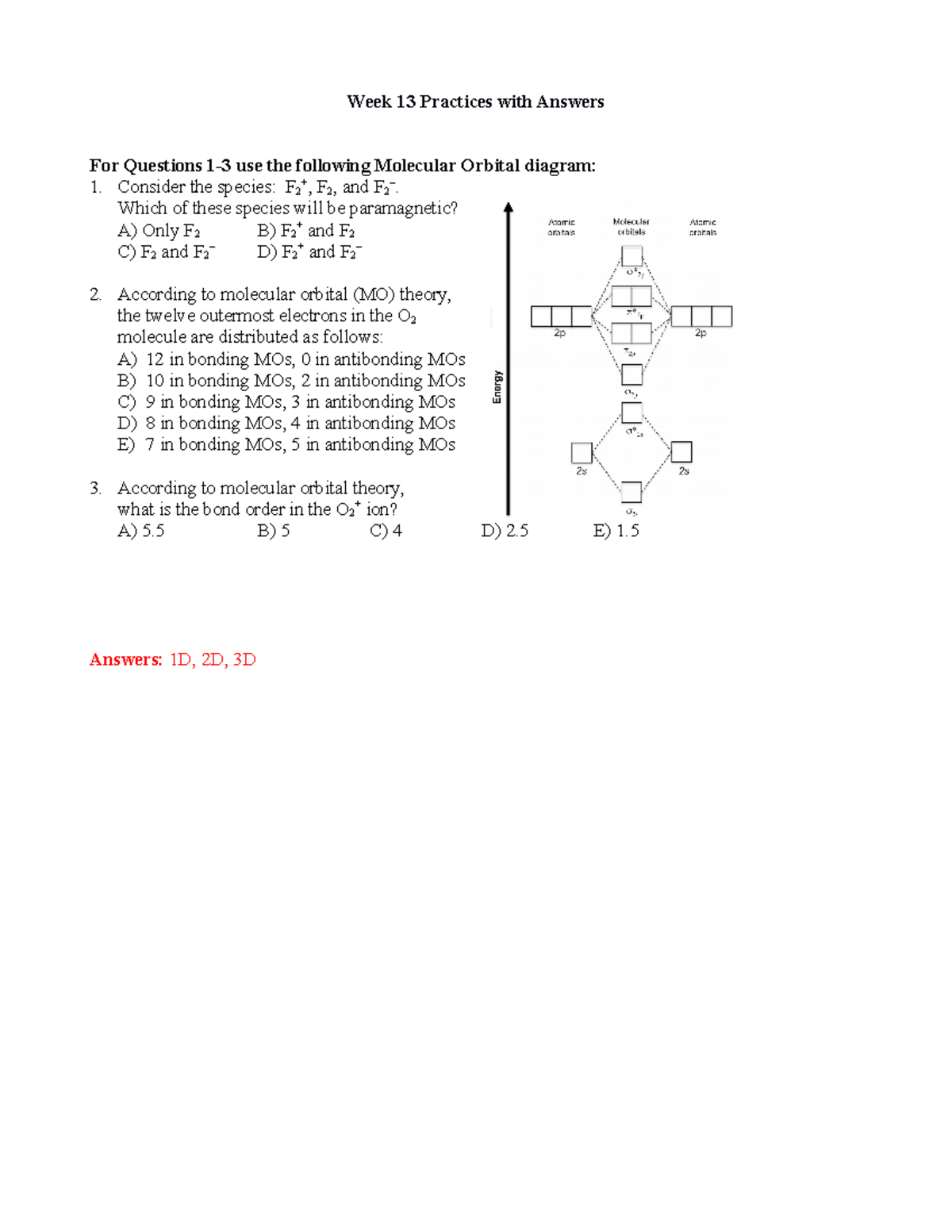
43 f2- molecular orbital diagram
February 3, 2018 - Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading. So, the hybridization here is sp3d. Two hybrid orbitals are used for sigma bond formation( single bond) in XeF2 (F-Xe-F). Molecular Orbital Diagram. If we go a little further into chemical bonding and hybridization, we get to know about the Molecular Orbital Theory, a concept of quantum mechanics.
Draw the molecular orbital diagram for the F2 ion, write the electron configuration, and calculate the... Draw the molecular orbital diagram for the F2 ion, write the electron configuration, and calculate the bond order for the F2 ion. Is the F2-ion stable?
F2- molecular orbital diagram
The HF involves one electron of H and an unpaired electron from a 2p orbital of F. As per the molecular orbital diagram, there is no unpaired electron in the hybridised orbital, hence it is diamagnetic. Which CO bond is the shortest? In CO,C−O bond gets triple bond character in one of the resonating structures. July 3, 2017 - (sigma_(2s))^2 (sigma_(2s)^"*")^2 (sigma_(2p_z))^2 (pi_(2p_x))^2 (pi_(2p_y))^2 (pi_(2p_x)^"*")^2 (pi_(2p_y)^"*")^2 Recall that there are orbital mixing effects for homonuclear diatomic molecules that decrease from left to right until "N"_2 (inclusive), which gives rise to a molecular orbital ... 15 F2 Molecular Orbital Diagram. We assume that the electrons would fill the molecular orbitals of molecules like electrons fill atomic we will use this diagram to describe o2, f2, ne2, co, and no. The lowest energy unoccupied molecular orbital is 2p_ (sigma), so that is where the extra electron will be added.
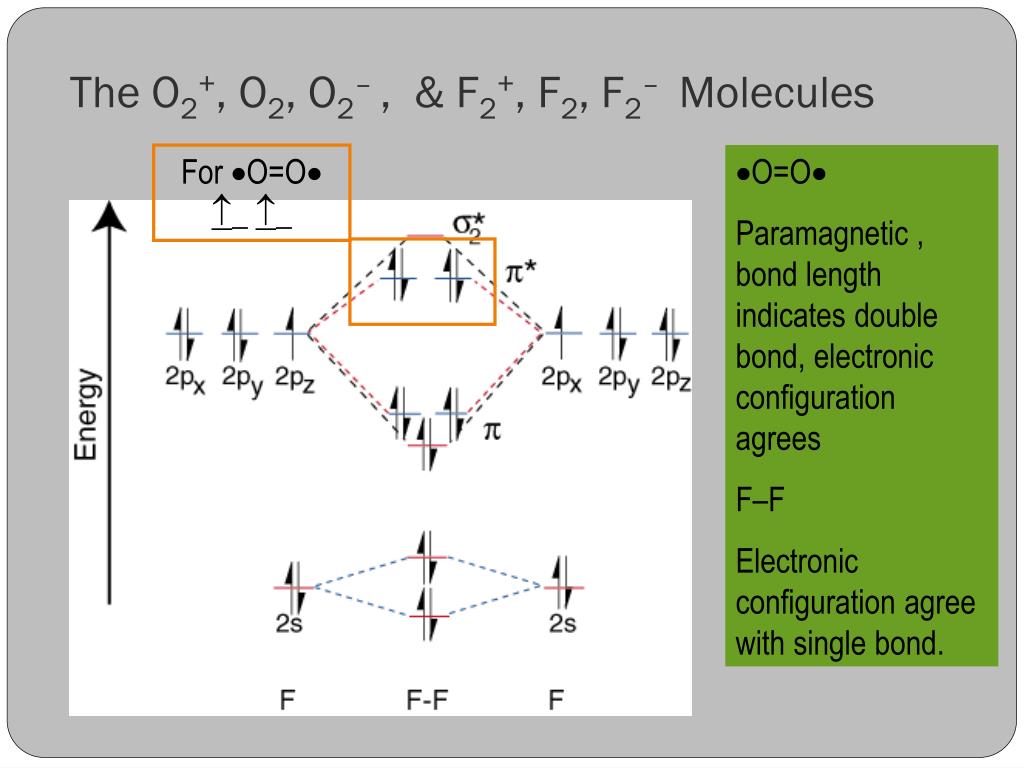
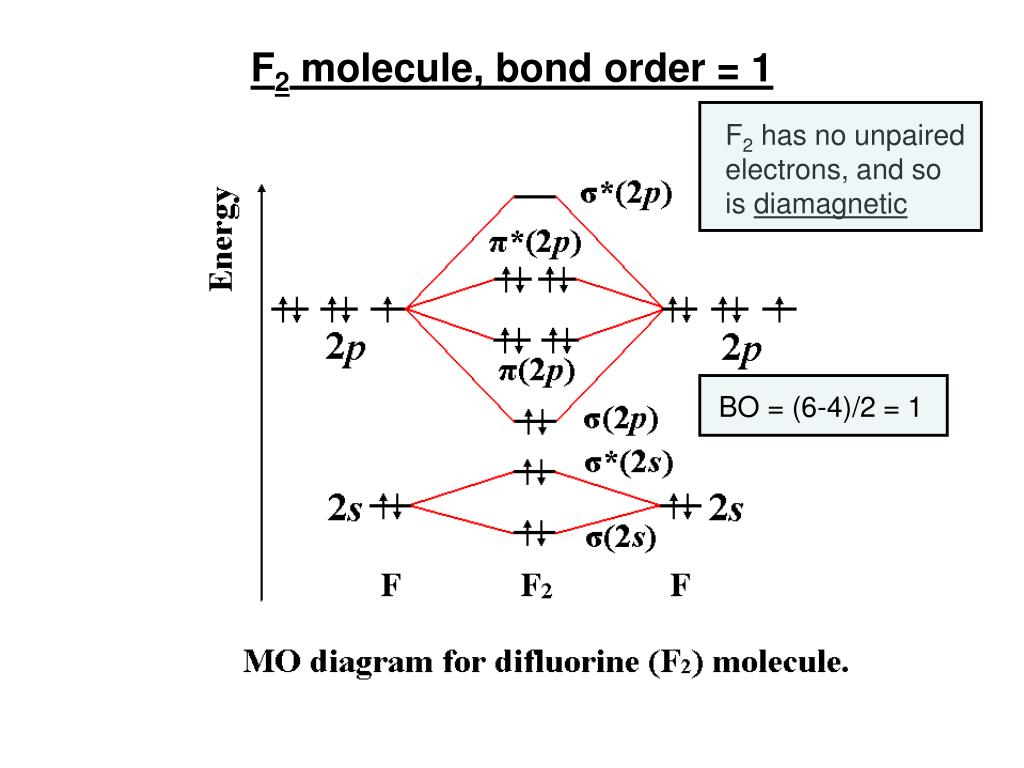
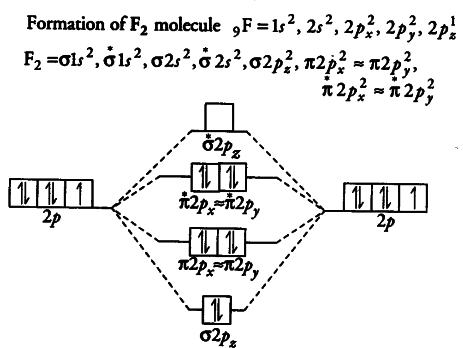
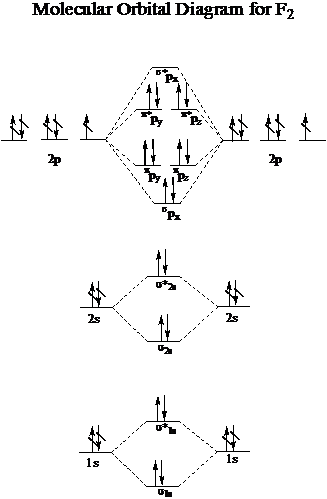
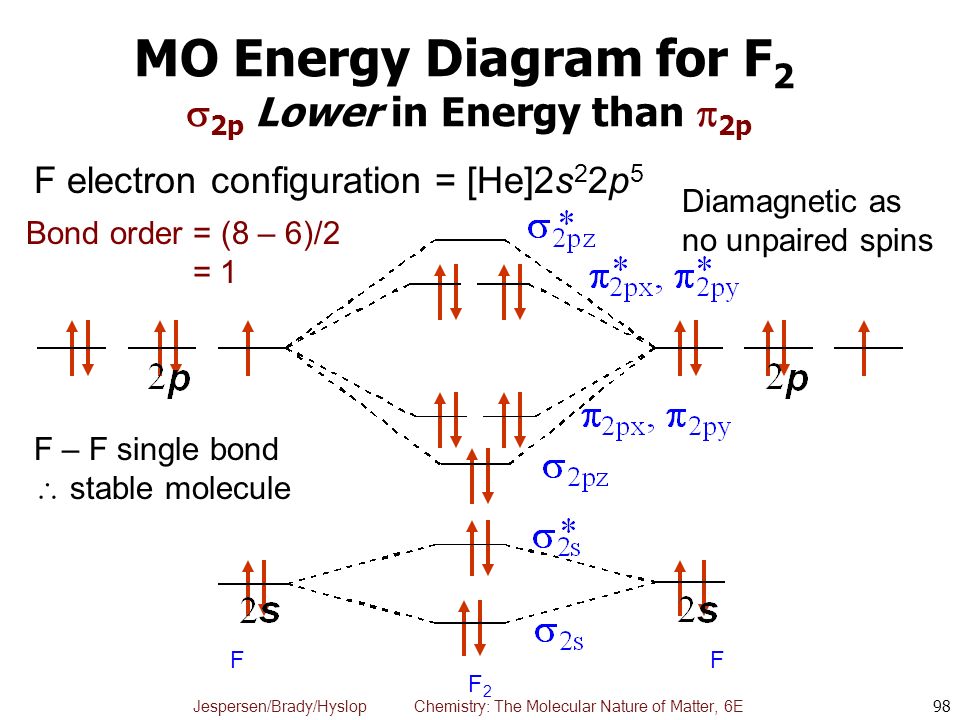
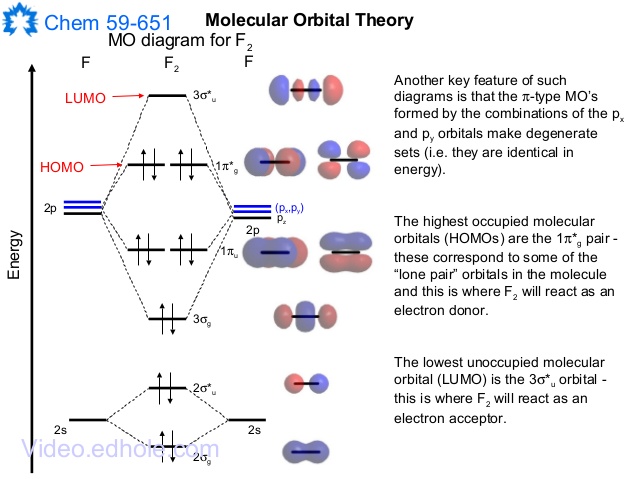
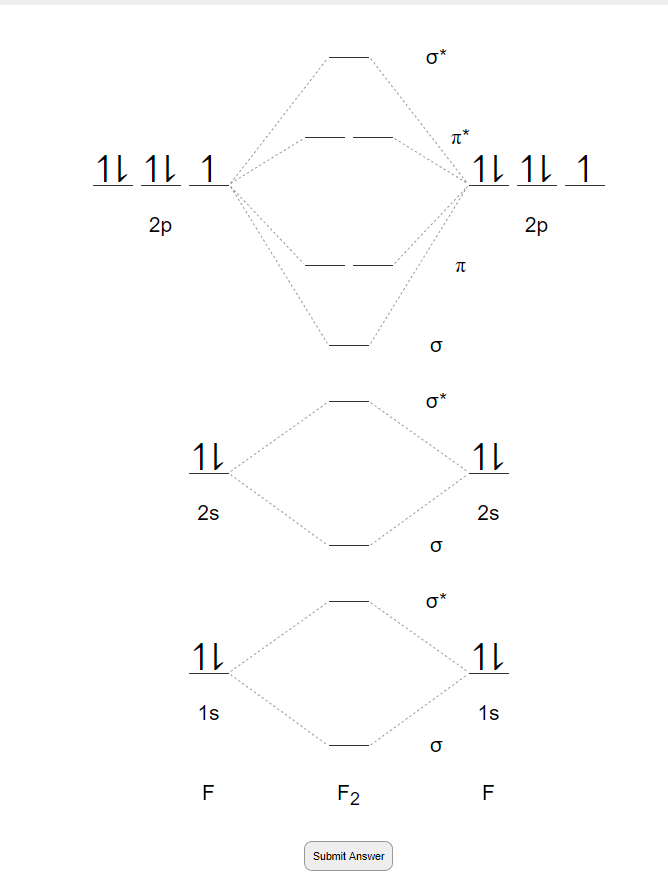
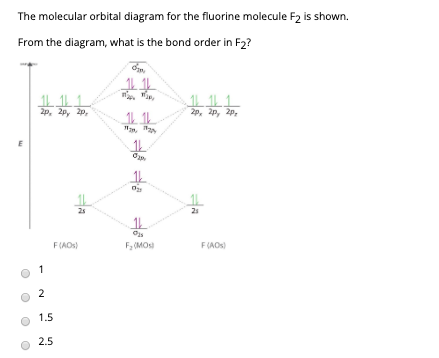
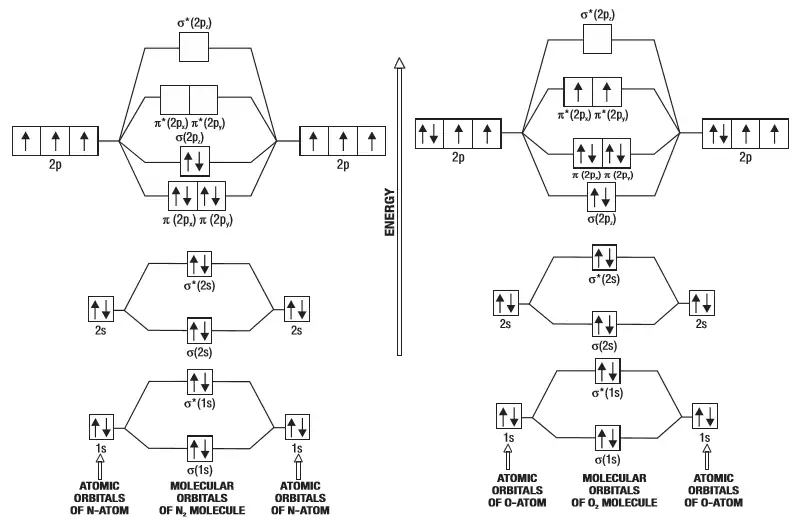
F2- molecular orbital diagram. Therefore, the bond order the O+2 is 2.5. There are 10 bonding electrons (including molecular orbitals created by the 1s orbitals.) and also 7 nonbonding electrons. Therefore, the bond order O−2 is 1.5. Which is an ext stable O2 or O2+? 1 Answer. O2+ is much more stable 보다 O2-. June 12, 2018 - Below is a molecular orbital diagram for a fluorine molecule. ... The highest occupied molecular orbitals (HOMOs) are antibonding, and so is the lowest unoccupied molecular orbital (LUMO). (i) Formation of F, molecule: Fluorine atom has one half-filled atomic orbital. Therefore, two atoms of fluorine combine to form the fluorine molecule. Formation of F 2 molecule (ii) Formation of HF molecule: HF molecule is formed as a result of the combination of half-filled orbitals belonging to hydrogen and fluorine. Formation of HF molecule Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
August 15, 2020 - A simple approach to molecular orbital (MO) theory for heterogeneous diatomic molecules is to show the energy level diagram. The MO energy levels can be worked out following these steps: The _valence shell configuration is 1s2 2s2. Two atomic orbitals joined to form a molecular orbital with a bonding, non-bonding and antibonding orbital. I Be _ 1s2 2s2 has 2 bonding and 2 antibonding orbitals. Order of bonds = 1/2 (b.oab.o) = 1/2 (2-2) = 0. So b.o from Be = 0. In this context, what is the binding order for be2 -? Answer: The bond order of He2(+) is 0.5. The helium molecule ion, He2(+), has altogether three electrons. Out of these three electrons, 2 occupy the lower bonding molecular orbital (MO) and the remaining one occupy the higher antibonding molecular orbital. Bond order = 1/2( no. of electrons in ... Learning Strategies No it is not paramagnetic.O2^2- has 2 electrons more than O2.Pi 2p molecular orbitals get completely filled hence it is diamagnetic. Fundamentals; 1. Textbook solution for Chemistry & Chemical Reactivity 9th Edition John C. Kotz Chapter 9.3 Problem 3RC. A O22- ion is: a. paramagnetic.
Solutions for Chapter 11Problem 32E: For each of the species C2+, O2−, F2+, and NO+,(a) Write the molecular orbital diagram (as in Example).Example(b)Determine the bond order, and state whether you expect the species to be stable or unstable.(c) Determine if the species is diamagnetic or ... August 15, 2020 - Molecular orbitals (MO) are constructed from atomic orbitals. In O2 and F2, there is a crossover of the sigma and the pi ortbials: the relative energies of the sigma orbitals drop below that of the pi orbitals'. Information from the MO diagram justify O2's stability and show that it's bonding ... Molecular Orbital Diagram Of C2, N2 , O2 ,F2 Updated On: 2-4-2021. To keep watching this video solution for FREE, Download our App. Join the 2 Crores+ Student community now! Watch Video in App Continue on Whatsapp. This browser does not support the video element. 2.4 k . 1.7 k . Answer. Question: Use Molecular Orbital Theory To Determine Whether He2 Or He2+ Is More Stable. These properties can be explained by the molecular orbital diagram of BN". The bond order of two suggests that the oxygen molecule is stable. Correct option (a) O-2. Diamagnetic Metals + properties give you a broad overview of these metals from multiple angels.
In the molecular orbital theory, bond length, bond strength, bond order, and magnetic properties of different molecules plays an important role. Bond order is just several electrons that are present between two atoms. One can calculate the bond order of any molecule by creating a molecular orbital diagram like calculate the bond order in h−2.. To create the molecular diagram, you can start ...
September 29, 2017 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!
Draw molecular orbital diagram for F2 molecule Also gives its electronic configuration bond order and magnetic property
The F2 molecular orbital diagram shows 4e- are in the highest energy antibonding (destabilizing) molecular orbitals resulting in a bond order = 1. Single bonds are easier to break and therefore more reactive. So the answer is yes.
Furthermore, How many bonds are in F2?, as fluorine has 9 electrons its valency is 1 to attain sability it makes one bond.so the answer is single bond. Finally, Is F2 2+ paramagnetic or diamagnetic?, F₂²⁺ is paramagnetic. From the molecular orbital electronic configuration, number of electrons present in pi orbitals present is equal to 2.
Explanation: Ne2^+ ought to have 19 electrons. Share with your friends. There are six carbons so no of sigma bonds 6×2= 12. The full molecular orbital electrons, which is 1 for or o2 f2 ne2 structure the case of bonds atoms. N2+ has 2 (5)-1 = 11 valence electrons. 1 Answer.
Answer to For each of the species C2+, O2−, F2+, and NO+,(a) Write the molecular orbital diagram (as in....

A Draw The Molecular Orbital Diagram Shown To Determine Which Of The Following Is Paramagnetic B 2 2 B2 C 2 2 B 2 2 And N 2 2 B Draw The Lewis Structures And Molecular Orbital Diagrams For
Incase of F2: Total electrons: 9 + 9 = 18 For F2 the ground state electron configuration would be: Favourite answer. But we have been asked the configuration of Iron in its Fe2+ form. Electrons in a molecule are said to occupy molecular orbitals. The main body of the table is a 18 × 7 grid.
In the XeF2 molecule, the three lone pairs are present as a result of the molecular geometry of XeF2 is line i.e. Linear For knowing the Bond Angle we can use AXE Notation, Where A is the central atom that is xenon, X is the number of atoms bonded to the central atom in the Lewis Structure 2 atoms is bonded to the central atom X=2 and E is the ...
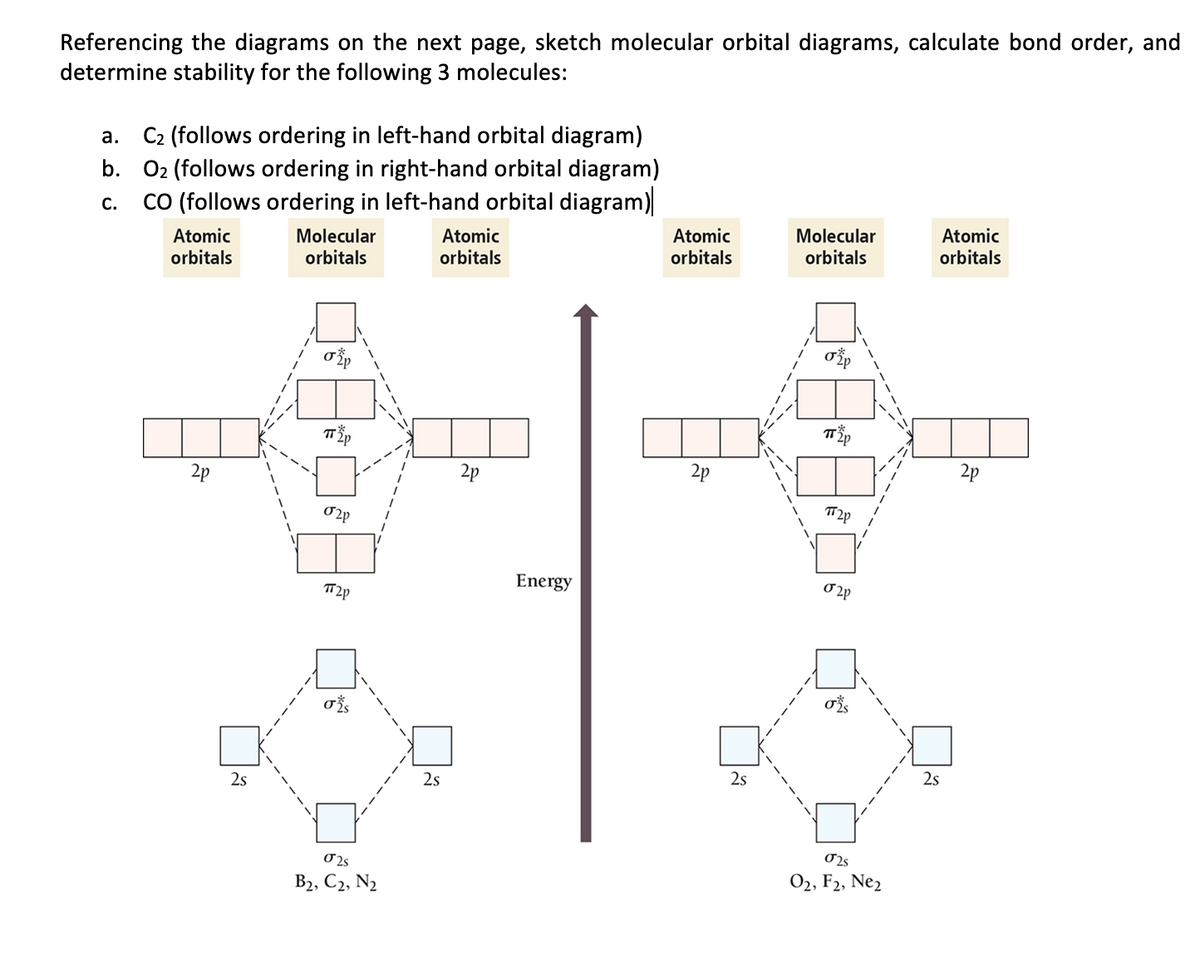
So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). ... In F2 the bonding is pure covalent, with the bonding electrons shared equally between the two fluorine atoms.

Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11
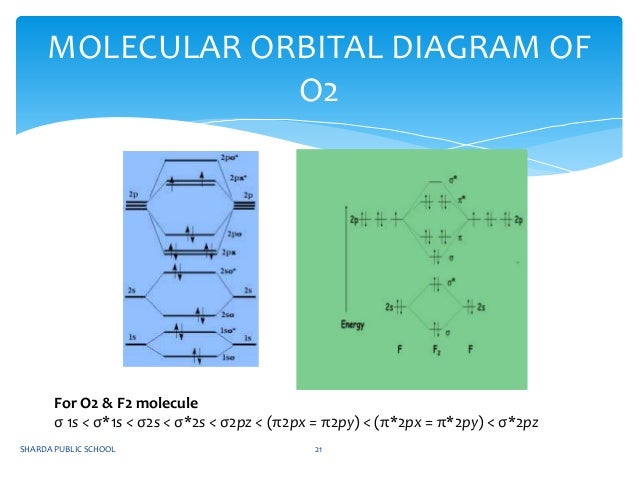
July 14, 2020 - N2 has the shortest bond because the bond order is 3 while O2 is 2 and F2 is 1. ... Draw the molecular orbital diagram for F2 with the atomic orbitals labeled and find the bond order.
April 5, 2018 - Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...

What Is The Energy Level Diagram Of N2 And F2 Chemistry Chemical Bonding And Molecular Structure 2442080 Meritnation Com
B2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of valence electrons of each boron atom = 3. In the formation of B2 molecule, three valence electrons of each boron atom i.e. 6 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
33 Molecular Orbital Diagram For F2 - Wiring Diagram Database. Share This: Facebook Twitter Google+ Pinterest Linkedin Print. About oiuetd Soratemplates is a blogger resources site is a provider of high quality blogger template with premium looking layout and robust design. di April 16, 2021.
November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
Answer to Please help draw the molecular orbital diagram for f2 and f2+ and the shortest bond to longest bond between f2, f2+, and...
November 9, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
1 Answer. 1. Electronic configuration of N atom is 1s2 2s2 2p3. 2. Electronic configuration of O atom is 1s2 2s2 2p4. 3. Electronic configuration of NO molecule is σ1s2 σ*1s2 σ2s2 σ*2s2 π2px2 π2py2 π2pz2 π*2px1. 4. Bond order = N b−N a 2 N b − N a 2 = 10−5 2 10 − 5 2 = 2.5.
Molecular Orbital Diagram Ne2 28.12.2018 28.12.2018 7 Comments on Molecular Orbital Diagram Ne2 Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.
May 4, 2020 - Solution for a. Using the molecular orbital diagram, calculate the bond order of F2+. Show show your work or give a brief explanation of the process. b. Do…

Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond
Molecular orbital diagram for carbon dimer c2. Molecular orbital diagram for n2 o2 c2 f2 also h2o. Fill from the bottom up with 8 electrons total. Mo diagram s can be used to deduce magnetic properties of a molecule and how they change with ionization. O2 2 Molecular Orbital Diagram Fabulous Electron Molecular Orbital. Molecular orbital diagram for c2.This video shows the mo diagrams of the c2 ...
Is f2 a bond order? Answer and Explanation: The bond order for fluorine gas is 1. This can be calculated by subtracting the number of anti-bonding electrons in the molecular orbitals from the What type of bond is f2? In F2 the bonding is pure covalent, with the bonding electrons shared equally between the two fluorine atoms.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
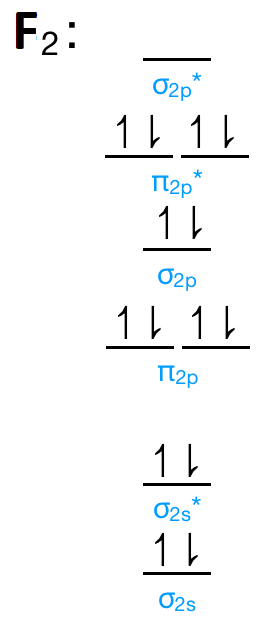
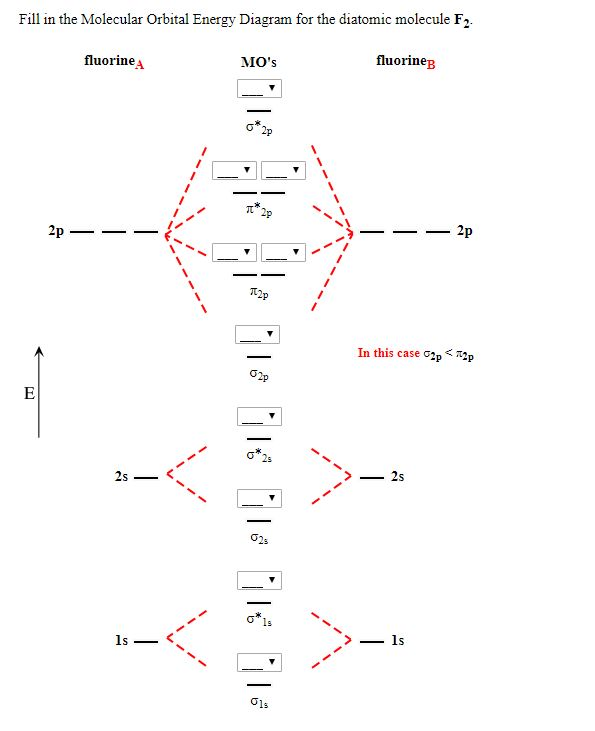
When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x 2, π2p y 2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
The case of F2 is a simple one because of the symmetry and diatomicity of the molecule. In more complex molecules (polyatomic and asymmetric), the extent of mixing and thus the contribution of individual atomic orbitals to form a particular molecular orbital depends on the relative energy alignment of the atomic orbitals. F2 Polarity
Ppt Chemistry 445 Lecture 4 Molecular Orbital Theory Of Diatomic Molecules Powerpoint Presentation Id 794473
Originally Answered: What is the link order that F2?In basic terms, since F has actually 7 valence electrons hence by sharing of electron with an additional F it develops a bond come fullfil its octate. Friend can also find the bond order using advancement Molecular orbital theory (MoT). As such Bond order that F2 is 1. You are watching: What is the bond order of f2
June 12, 2020 - Click here👆to get an answer to your question ✍️ 37. Draw molecular orbital diagram for F2 molecule. Also, give its electronic configuration, bond order and magnetic property. 138. Solve the following:
FREE Answer to Question 1) By drawing molecular orbital diagrams for B2, C2, N2, O2, and F2, predict which...
Arrange the following in order of decreasing stability. a blank molecular orbital diagram (part a 1 figure) has been provided to you. rank the fluorine species from most to least stable. to rank items as equivalent, overlap them. f2, f2+, f2-
15 F2 Molecular Orbital Diagram. We assume that the electrons would fill the molecular orbitals of molecules like electrons fill atomic we will use this diagram to describe o2, f2, ne2, co, and no. The lowest energy unoccupied molecular orbital is 2p_ (sigma), so that is where the extra electron will be added.
July 3, 2017 - (sigma_(2s))^2 (sigma_(2s)^"*")^2 (sigma_(2p_z))^2 (pi_(2p_x))^2 (pi_(2p_y))^2 (pi_(2p_x)^"*")^2 (pi_(2p_y)^"*")^2 Recall that there are orbital mixing effects for homonuclear diatomic molecules that decrease from left to right until "N"_2 (inclusive), which gives rise to a molecular orbital ...
The HF involves one electron of H and an unpaired electron from a 2p orbital of F. As per the molecular orbital diagram, there is no unpaired electron in the hybridised orbital, hence it is diamagnetic. Which CO bond is the shortest? In CO,C−O bond gets triple bond character in one of the resonating structures.
Use The Molecular Orbital Energy Level Diagram To Show That Cbse Class 11 Chemistry Learn Cbse Forum












0 Response to "43 f2- molecular orbital diagram"
Post a Comment