43 lennard jones potential diagram
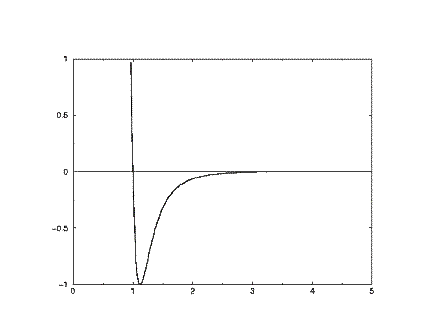
The Lennard-Jones potential (LJ) is a simple pair potential that can accurately model weak van der Waals bonds between noble gases, yielding reliable bond energies and bond lengths ( Lennard-Jones 1924 ). The LJ has the form. (1) V LJ(r) = 4ɛ[(σ γ)12 − (σ γ)6] ɛ. where r is the distance between any two atoms and ε and σ are ... where m and n are integers, and C n and C m are constants whose values depend on the depth of the energy well and the equilibrium separation of the two atoms' nuclei. Typically the 12-6 Lennard-Jones parameters (n =12, m =6) are used to model the Van der Waals' forces 1 experienced between two instantaneous dipoles.However, the 12-10 form of this expression (n =12, m =10) can be used to model ...
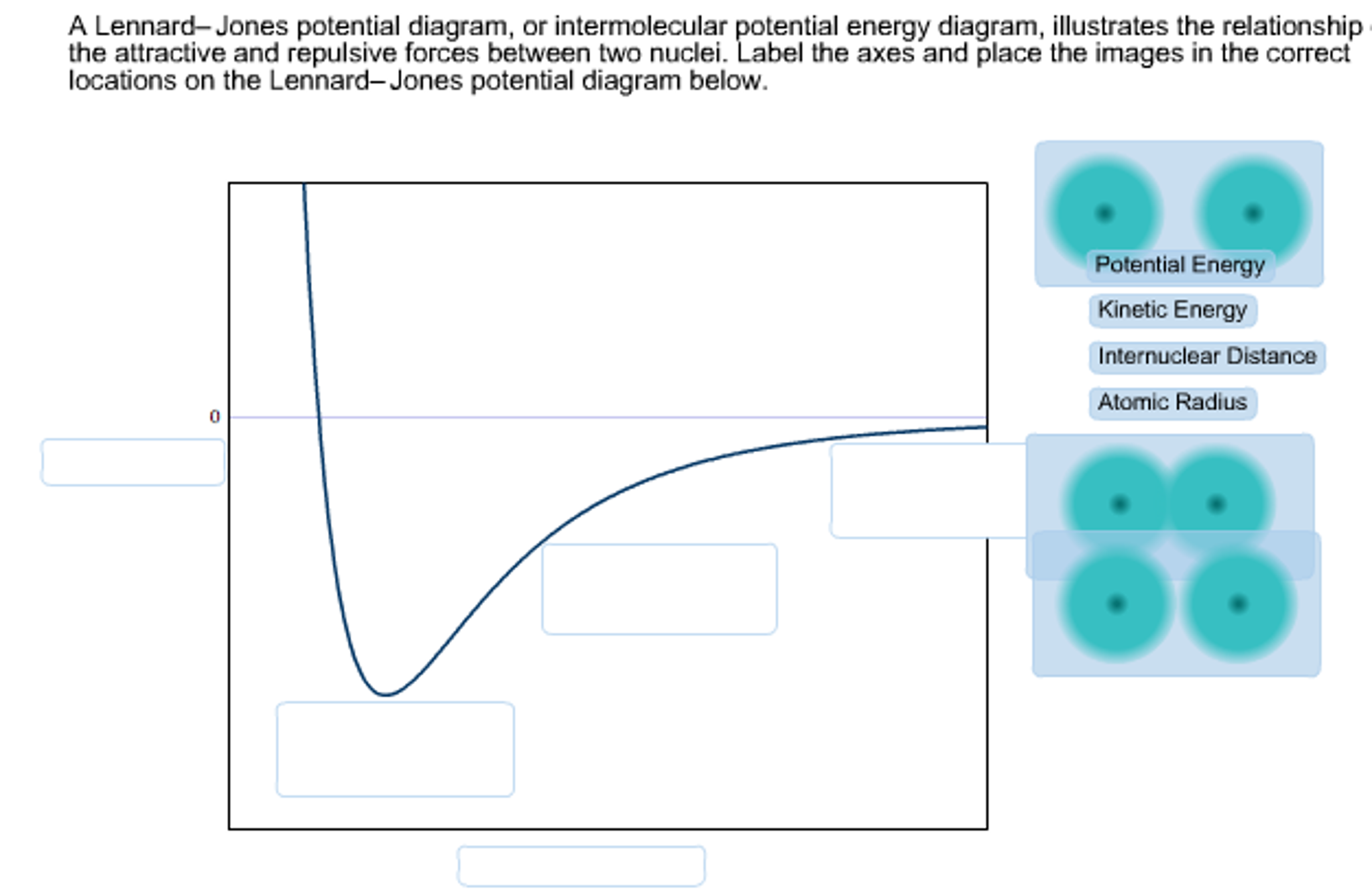
Lennard- Jones potential diagrams, also called intermolecular potential energy diagrams, illustrate the relationship between the potential energy of a molecule as the distance between the two nuclei changes. Select all of the true statements regarding Lennard-Jones potential diagrams from the list below. As the internuclear distance decreases ...
Lennard jones potential diagram
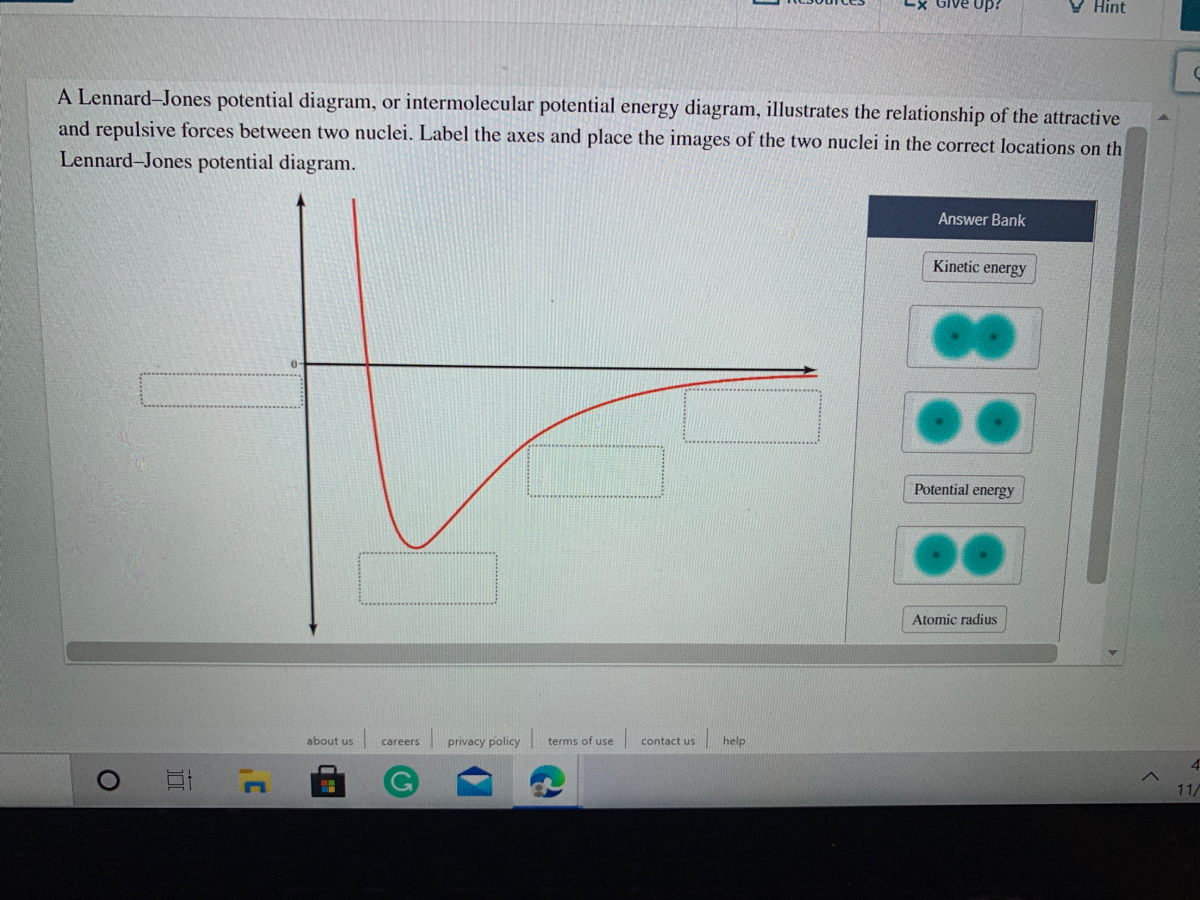
Lennard-Jones potential, it has been reported 15 that two different versions of the Clapeyron equation must be used below and above T=2.74 to maintain the stability of the integration. The starting point of T=2.74 was used for the high temperature GDI simulations without any further rigor-ous testing of the GDI series for the WCA potential. efficients of the Lennard-Jones potential in the fluid region of the phase diagram by equilibrium molecular-dynamics simu-lations using time-correlation function theory. In general, several theoretical approaches are available to calculate transport coefficients from a knowledge of the intermolecular forces. For example, kinetic theory as de- A Lennard-Jones potential diagram, or intermolecular potential energy diagram, illustrates the relationship of the attractive and repulsive forces between two nuclei. Label the axes and place the images in the correct locations on the Leonard-Jones potential diagram below. Leonard-Jones potential diagrams, also called intermolecular potential ...
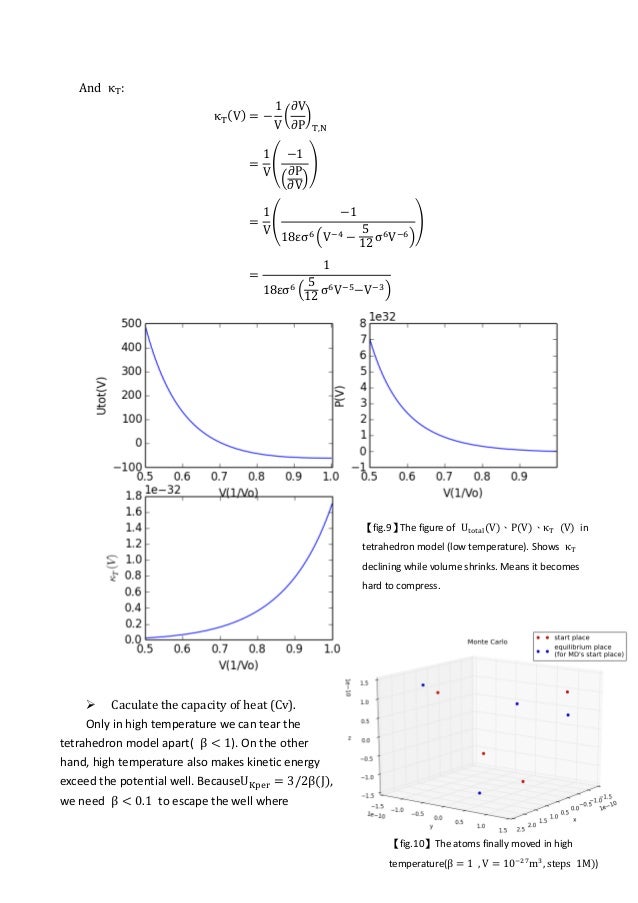
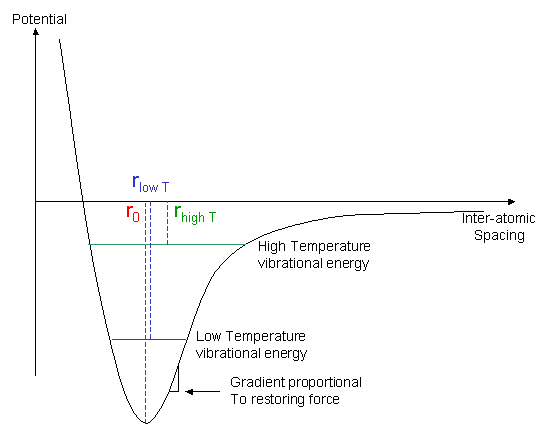
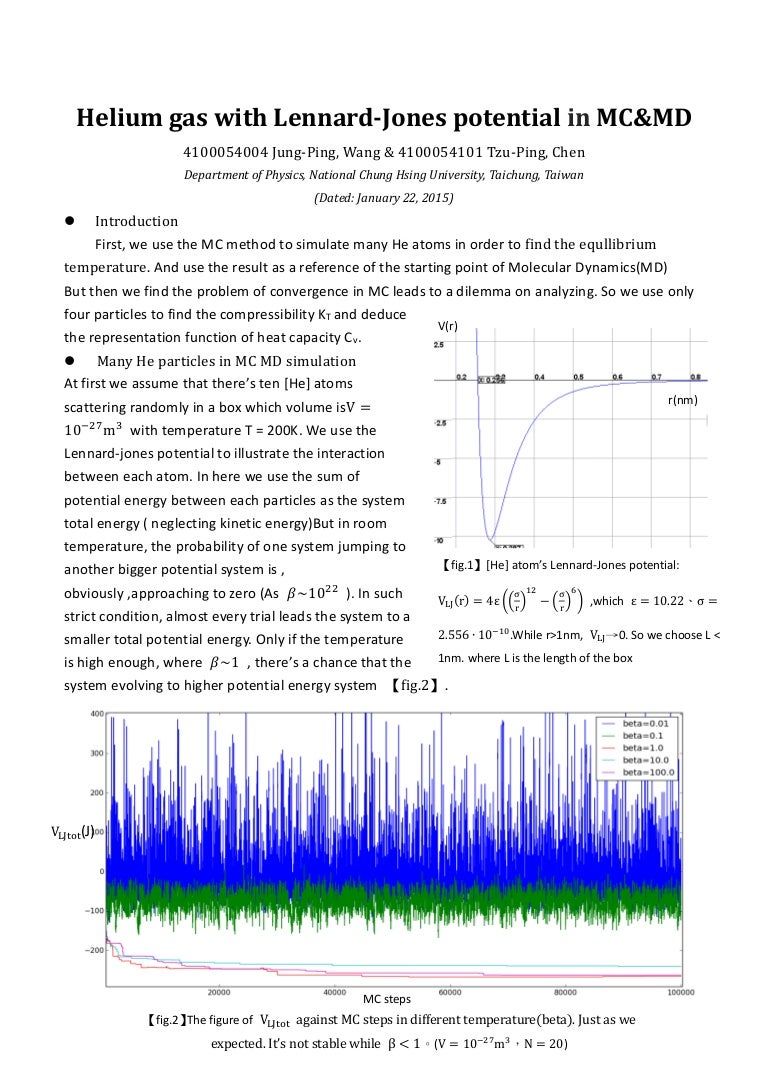
Lennard jones potential diagram. polynomial, and the convex envelope construction method was used to determine the phase diagram. The Lennard-Jones intermolecular potential is given by u ij(r) = 4 ij σ ij r 12 − σ ij r 6, (1) where u ij is the potential energy of interaction between particles i and j, r the distance between particles i and j, ij the Lennard-Jones ... Forget the difference between the Harmonic and Morse (I think I get that), It's more what the horizontal lines mean and what the boundary line means. I know that vibrational energy is quantized and I can rationalize the shape of the Lennard-Jones potential but I don't understand how they're combined in this diagram. Equation 1: The Lennard-Jones potential. The LJP model is composed of two components: the repulsive (σ/r)¹² and the attractive term (σ/r)⁶, which respectively denote repulsive and attractive forces.The parameter r is the distance between the two atoms (in Å units);. σ is a length scale representing the distance at which the intermolecular potential between the two atoms is = 0 (in Å ... Lennard-Jones model Frank H. Stillinger Agere Systems, Murray Hill, New Jersey 07974 and Princeton Materials Institute, Princeton University, Princeton, New Jersey 08544 Received 21 May 2001; accepted 26 June 2001 High-accuracy lattice sums have been evaluated for the Lennard-Jones 12-6 pair potential, without
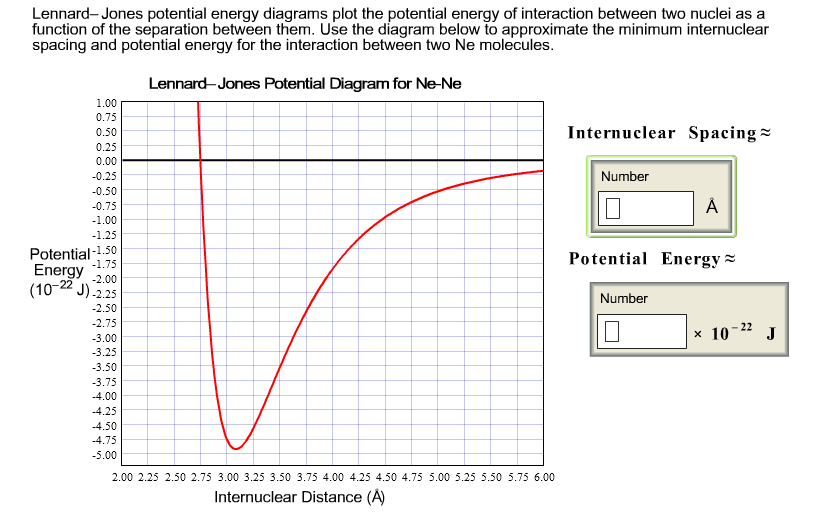
Related reading. John Edward Lennard-Jones "On the Atomic Fields of Helium and Neon", Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character 107 pp. 157-170 (1925); J. G. Powles and J. L. F. Abascal "The structure of liquid neon: an anomaly resolved", Journal of Physics C: Solid State Physics 16 L441 (1983) The Lennard-Jones potential energy function describes the force between two neutral atoms in a molecule. x is the atomic separation distance. Summary 201 Example 7.9 Force and Energy on an Atomic Scale The potential energy associated with the force between two neutral atoms in a molecule can be modeled by the LennardÐJones potential energy ... Lennard-Jones potential energy diagrams plot the potential energy of interaction between two atoms as a function of the separation between them. Use the diagram to approximate the optimum internuclear spacing and Lennard-Jones potential diagram for Ne-Ne 1.00 - 0.50- 0.00 potential energy for the interaction between two Ne atoms. Abstract. Cluster formation in a two-dimensional Lennard-Jones system under different conditions of temperature (𝑇) and particle concentration (𝑐) has been studied using the Monte-Carlo method with the introduction of real thermal motion of the constituent particles through a modification of the conventional Metropolis algorithm.The 𝑐-𝑇 phase diagram determined from the study of ...
The gas-liquid phase transition of the three-dimensional Lennard-Jones particles system is studied by molecular dynamics simulations. The gas and liquid densities in the coexisting state are determined with high accuracy. The critical point is determined by the block density analysis of the Binder parameter with the aid of the law of rectilinear diameter. Lennard-Jones diagram After collision of the molecule with the surface, there are two successive sequences before adsorption [1, 17] The molecule adheres at the surface with the formation of a precursor state. The molecule may or not be dissociated and after moves or diffuses over the The adsorption can be represented energetically through the Lennard-Jones diagram [17], displaying the ... @article{osti_5799298, title = {Phase diagram of a Lennard-Jones solid}, author = {Choi, Y and Ree, T and Ree, F H}, abstractNote = {A phase diagram of a Lennard-Jones solid at [ital kT]/[epsilon][ge]0.8 is constructed by our recent perturbation theory. It shows the stability of the face-centered-cubic phase except within a small pressure and temperature domain, where the hexagonal-close ... Download scientific diagram | Lennard-Jones potential U(r) , and internal force F(r) . from publication: Mechanical Behavior of Nano Structures Using Atomic-Scale Finite Element Method (AFEM ...
Lennard-Jones Potential. The LJ potential requires the assignment of two parameters for each atom type in a force field, which describe how favorable the interatomic interaction is and at what distance the energy minimum occurs. From: Progress in Molecular Biology and Translational Science, 2020. Related terms: Hydrogen Bond; Molecular Mechanics
Lennard-Jones potential (also referred to as the L-J potential, 6-12 potential or, less commonly, 12-6 potential) is a simple mathematical model that represents this behavior. It was proposed in 1924 by John Lennard-Jones. The L-J potential is of the form V(r) = 4 [(˙ r) 12 (˙ r) 6] where is the depth of the potential well and ˙is the ( nite)
I am using the Lennard Jones potential with 108 particles and Berendsen thermostat and barostat to fix the parameter. In order to observe phase transition I plot the radial distribution function (RDF) and also density versus temperature graph for various fixed temperature. However it is hard to differentiate liquid and gaseous phase from RDF.
The Lennard-Jones potential (also termed the LJ potential or 12-6 potential) is an intermolecular pair potential.Among the intermolecular potentials, the Lennard-Jones potential is the potential that has been studied most extensively and most thoroughly.It is considered an archetype model for simple yet realistic intermolecular interactions. The Lennard-Jones potential models soft repulsive ...
Lennard-Jones potential diagrams, also called intermolecular potential energy diagrams, illustrate the relationship between the potential energy of a molecule as the distance between the two nuclei changes. Select all of the true statements regarding Lennard-Jones potential diagrams. 1.
The Lennard-Jones potential is a function of the distance between the centers of two particles. When two non-bonding particles are an infinite distance apart, the possibility of them coming together and interacting is minimal. For simplicity's sake, their bonding potential energy is considered zero. However, as the distance of separation ...
Gibbs ensemble simulations are used to calculate part of the phase diagram of the Lennard‐Jones fluid. The phase diagram turned out to be very sensitive to the details of how the tail of the Lennard‐Jones potential is taken into account in the simulations.
Adopting temperature dependent interaction parameters in the Lennard-Jones potential, the vapour-liquid phase diagram of methane was produced using NVT Gibbs Ensemble Monte Carlo technique.
I have a Lennard-Jones diagram here where I can't figure out, what is on the x-axis. G. Ertl, Angew. Chem. Int. Ed. 2008, 47. We are looking at a diagram for a molecule $\mathrm A_2$ composed of two $\mathrm A$ atoms that comes into close contact with a surface.

Pdf Canonical Dual Theory Applied To A Lennard Jones Potential Minimization Problem Semantic Scholar
Jones potential, the full Lennard-Jones potential is meta- stable and therefore the validity of the Nicolas EOS at those conditions is questionable. The effect of shifting the Lennard-Jones potential on the phase diagram is also demonstrated in Fig. 2, which shows that the shift in potential lowers critical
The Lennard-Jones intermolecular pair potential is a special case of the Mie potential and takes its name from Sir John Edward Lennard-Jones.The Lennard-Jones model consists of two 'parts'; a steep repulsive term, and smoother attractive term, representing the London dispersion forces .The Lennard-Jones potential is probably the most extensively studied model potential today .
A Lennard-Jones potential diagram, or intermolecular potential energy diagram, illustrates the relationship of the attractive and repulsive forces between two nuclei. Label the axes and place the images in the correct locations on the Leonard-Jones potential diagram below. Leonard-Jones potential diagrams, also called intermolecular potential ...
efficients of the Lennard-Jones potential in the fluid region of the phase diagram by equilibrium molecular-dynamics simu-lations using time-correlation function theory. In general, several theoretical approaches are available to calculate transport coefficients from a knowledge of the intermolecular forces. For example, kinetic theory as de-
Lennard-Jones potential, it has been reported 15 that two different versions of the Clapeyron equation must be used below and above T=2.74 to maintain the stability of the integration. The starting point of T=2.74 was used for the high temperature GDI simulations without any further rigor-ous testing of the GDI series for the WCA potential.
Frontiers Transport Properties Of The Simple Lennard Jones Spline Fluid I Binary Scattering And High Accuracy Low Density Transport Coefficients Physics

Introduction Scanning Force Microscopy Sfm Soft Matter Physics Division University Of Leipzig Molecular Dynamics Soft Matter Physics

The Lennard Jones Potential Why The Art Of Molecular Dynamics Is So By Luca Zammataro Towards Data Science

The Extended Lennard Jones Potential Energy Function A Simpler Model For Direct Potential Fit Analysis Sciencedirect

Why Do Bonds Form Energy Of Two Separate H Atoms Lennard Jones Potential Energy Diagram For The Hydrogen Molecule Forces Involved We Understand That Ppt Download





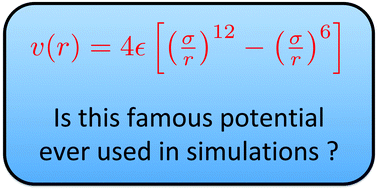











0 Response to "43 lennard jones potential diagram"
Post a Comment