44 mo diagram for n2
Nov 12, 2017 · 2 answersLet me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first ...What is the bond order of N2-? - QuoraJun 21, 2018Why is the molecular orbital diagram for O₂ different from N₂?Aug 1, 2019What is the molecular orbital diagram of O2 and F2? - QuoraMar 12, 2017How does the Lewis structure of N2 related to the molecular ...Apr 30, 2017More results from www.quora.com Molecular Orbital Diagram of N2. Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
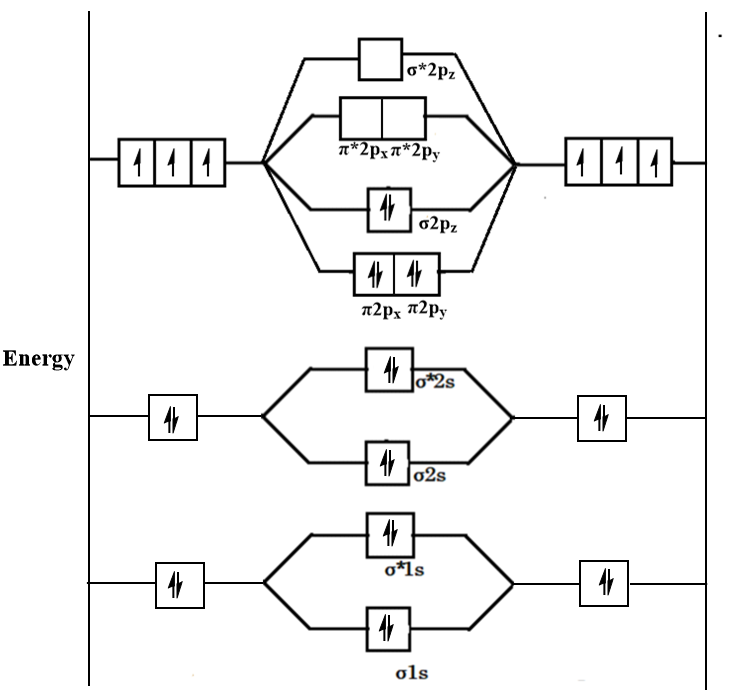
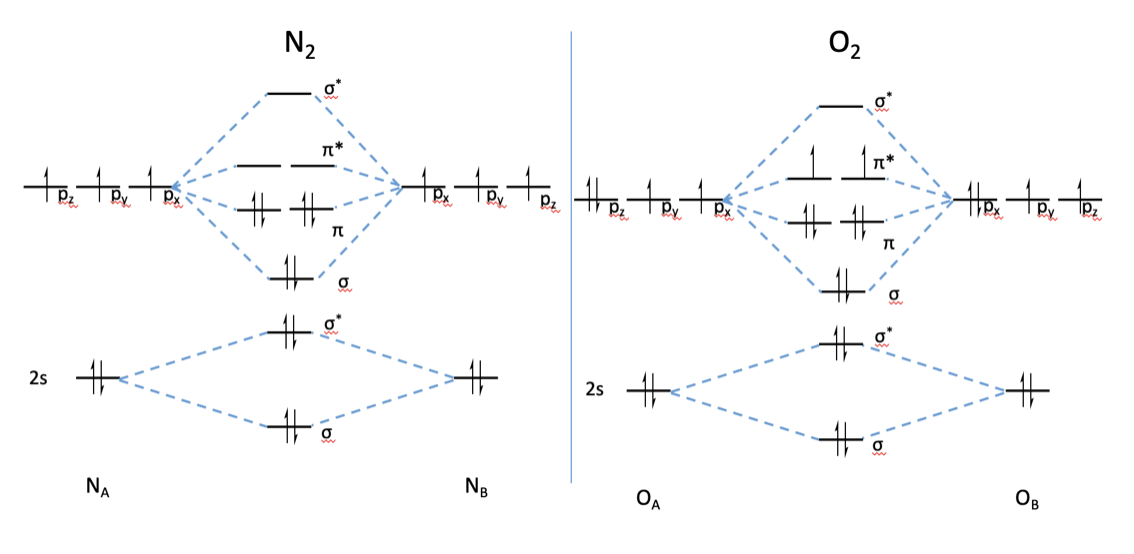
What is the MO diagram and bond order for N2 ( in Urdu / Hindi) Nitrogen (N 2 ) molecule: Nitrogen atom has electronic configuration 1s2, 2s2, 2p3.

Mo diagram for n2
Draw the molecular orbital diagram of N2. Also find its bond order and magnetic · Asked by Topperlearning User | 13th Jun, 2016, 02:45: PM. Expert Answer: ... Molecular orbital diagram for n2. Then just fill the. One is for the elements up to nitrogen. For the second period elements the 2s and 2p orbitals are important for mo considerations. Since the s h h orbital shows a decrease in bonding between the two nuclei. Perpendicular to these in the yz plane the 2py orbitals on each atom combine to make ... N2 molecular orbital energy level diagram also has the following tags. Three filled bonding orbitals. Mo diagrams for diatomic molecules chapter 5 friday october 9 2015. Here is the full molecular orbital diagram for n2. N2 molecular orbital energy level diagram with description. Now we add the 10 electrons 5 from each nitrogen atom. This image ...
Mo diagram for n2. There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start... Molecular#Orbital#Diagram#Nitrogen #Molecule#Chemistry #Class11 #NEET #JEE #MDCAT #ECAT ...Mar 2, 2021 Why is the MO diagram different for N2 and N2-? I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For $\\ce{N2}$ the orbitals in increasing energy are: because it has 14 electrons. For $\\ce{N2-}$ there are 15 electrons. On a very general basis, electrons are not assigned to individual bonds between atoms, but they move under the influence of the nuclei in the whole molecule. Molecular orbital theory is a method for describing the electronic structure of the molecule. Now, let us draw the molecular orbital diagram of ${N_2}$ .
Molecular Orbital Diagram Ne2 28.12.2018 28.12.2018 7 Comments on Molecular Orbital Diagram Ne2 Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules ... Molecules with Similar Molecular Orbital Diagrams Molecules and ions formed from 2 boron atoms or from 2 carbon atoms have molecular orbitals diagrams of the same sort as N 2. Diatomic molecules made up of two different atoms also have molecular orbital diagrams very similar to that of N 2.When the electronegativity of one atom is lower than the other, the more electronegative atom's orbitals ... 14+ N2 Mo Diagram. With mo diagrams, we can predict the number of bonds in diatomic molecules. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2). N2 2 Molecular Orbital Diagram — UNTPIKAPPS from www.untpikapps.com Thus if we know…
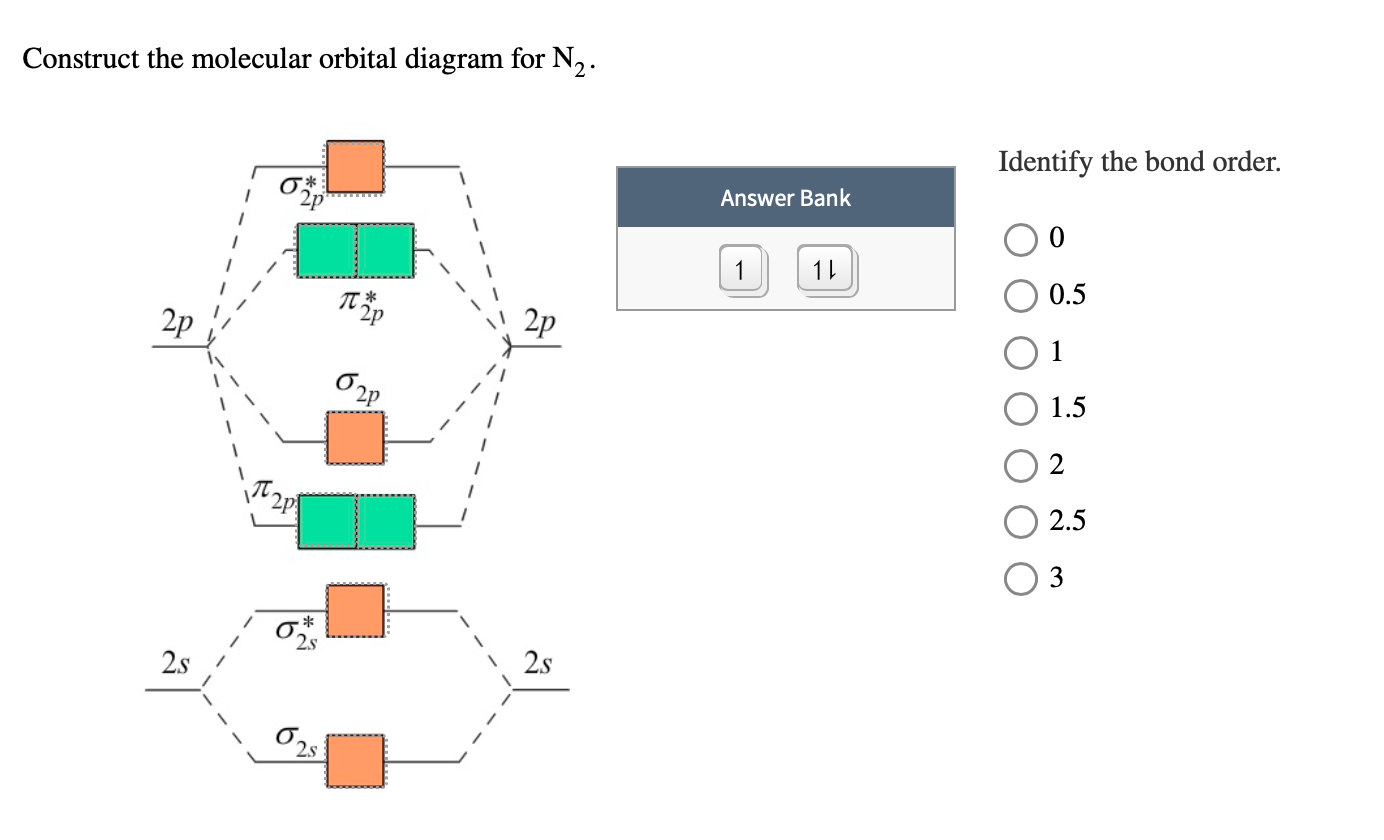
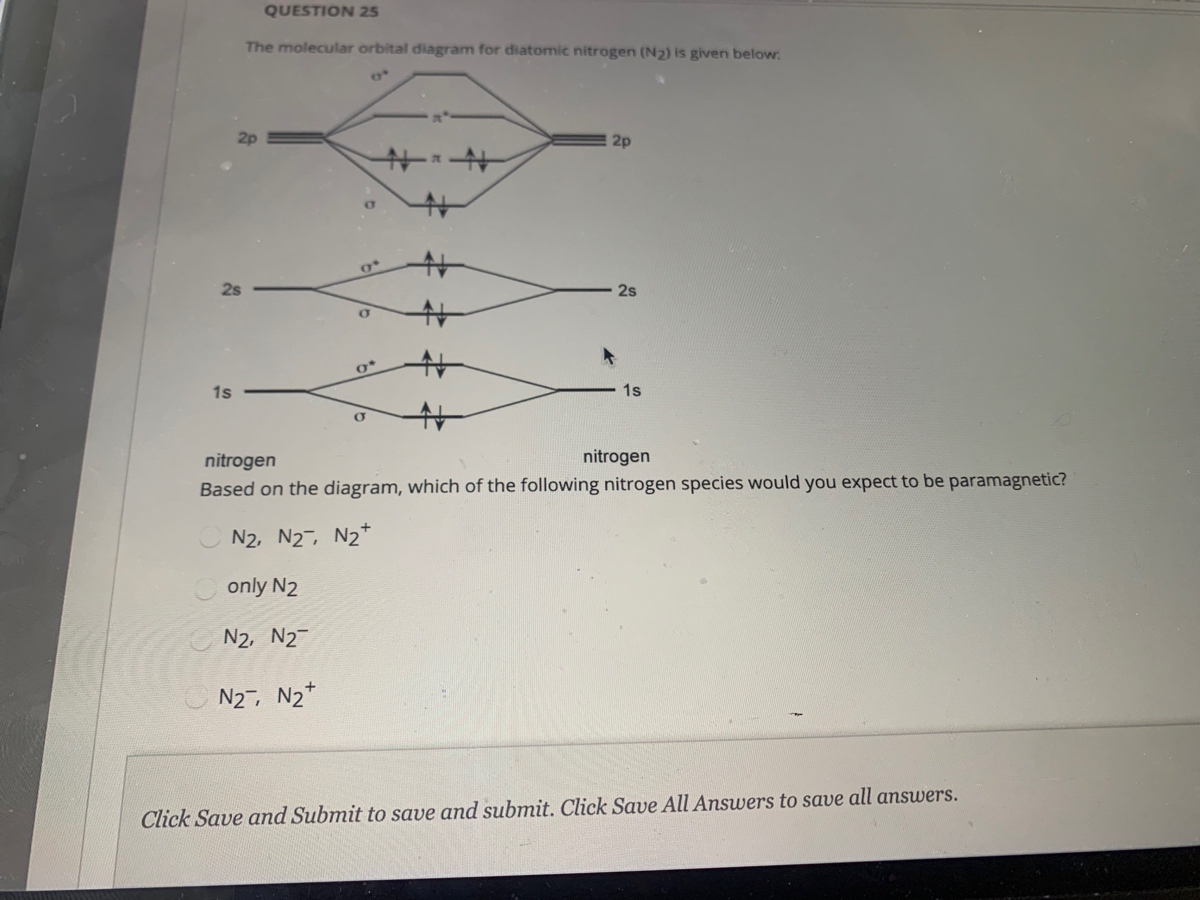
molecular orbital energy-level diagram for the NO molecule. We assume that orbital order is the same as that for N2. The bond order is 2.5. Figure 9.42: The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure 9.43: A partial molecular orbital energy-level diagram for the HF molecule. Molecular orbital (MO) diagram for N2 and N2^-Ask Question Asked 6 years, 3 months ago. Active 3 years, 10 months ago. Viewed 118k times 24 7 $\begingroup$ I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For $\ce{N2}$ the orbitals in ... Answer (1 of 4): Bond order of N2 is 3 it can be calculated by bond order method and by some electron numbers 10 electrons - 1.0 11 electrons - 1.5 12 electrons - 2.0 13 electrons - 2.5 14 electrons - 3 it is middle number in this series. As you go up or down it decreases 0.5. 15 electrons ... Draw the molecular orbital energy level diagram of N2 molecules. >>. Class 11. >> Chemistry. >> Chemical Bonding and Molecular Structure. >> Molecular Orbital Theory. >> Draw the molecular orbital ...
In a molecular orbital diagram, the diagram of molecular orbital energy levels is shown as horizontal lines. Degenerate orbitals (orbitals having the same energy) are shown side by side in these diagrams. Electrons are filled according to the Pauli Exclusion Principle. Basic structure of molecular orbital diagram for nitrogen is:
MO diagram of N2(2-) Ask Question Asked 6 years, 9 months ago. Active 4 years, 1 month ago. Viewed 22k times 11 $\begingroup$ In the MO diagram of $\ce{N2^2-}$, does s-p mixing happen to a significant degree to change the ordering of orbitals - $\pi_\mathrm{2p}$ orbital below $\sigma_\mathrm{2p}$? As it is isoelectronic with oxygen, I think ...
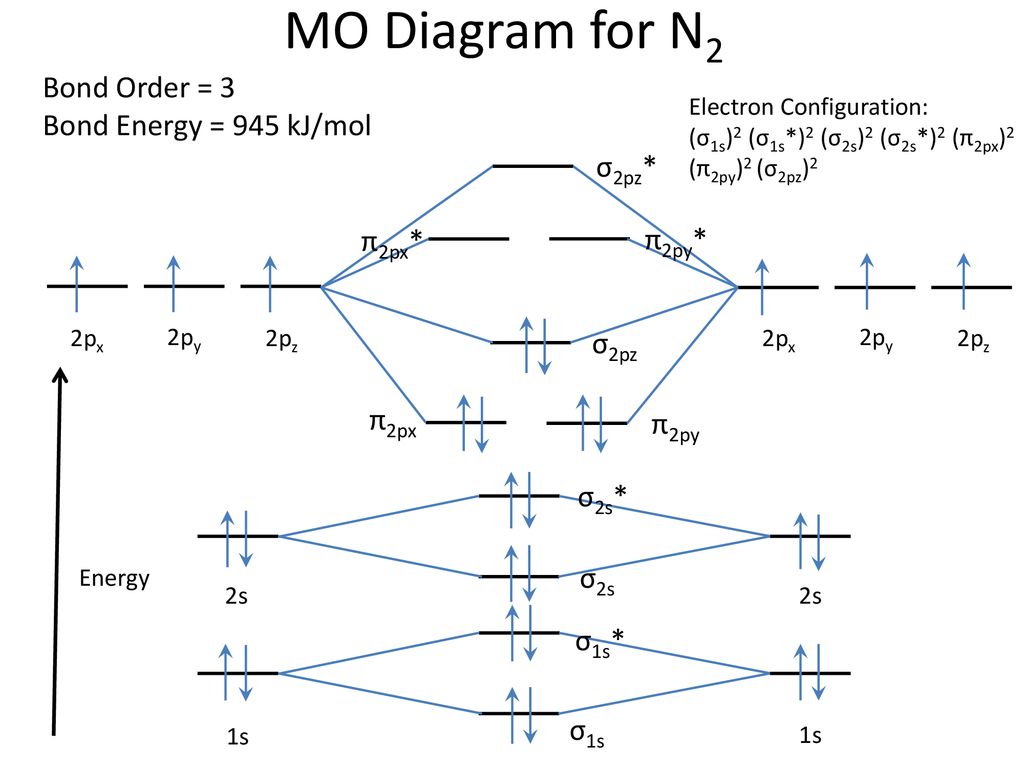
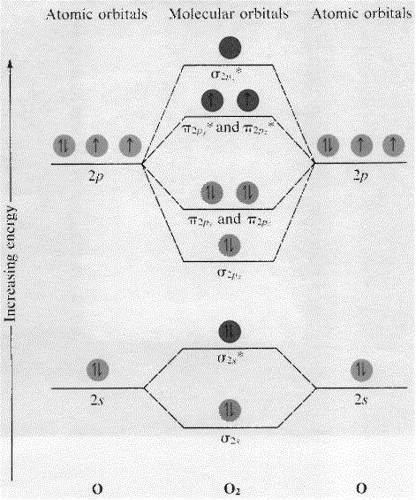
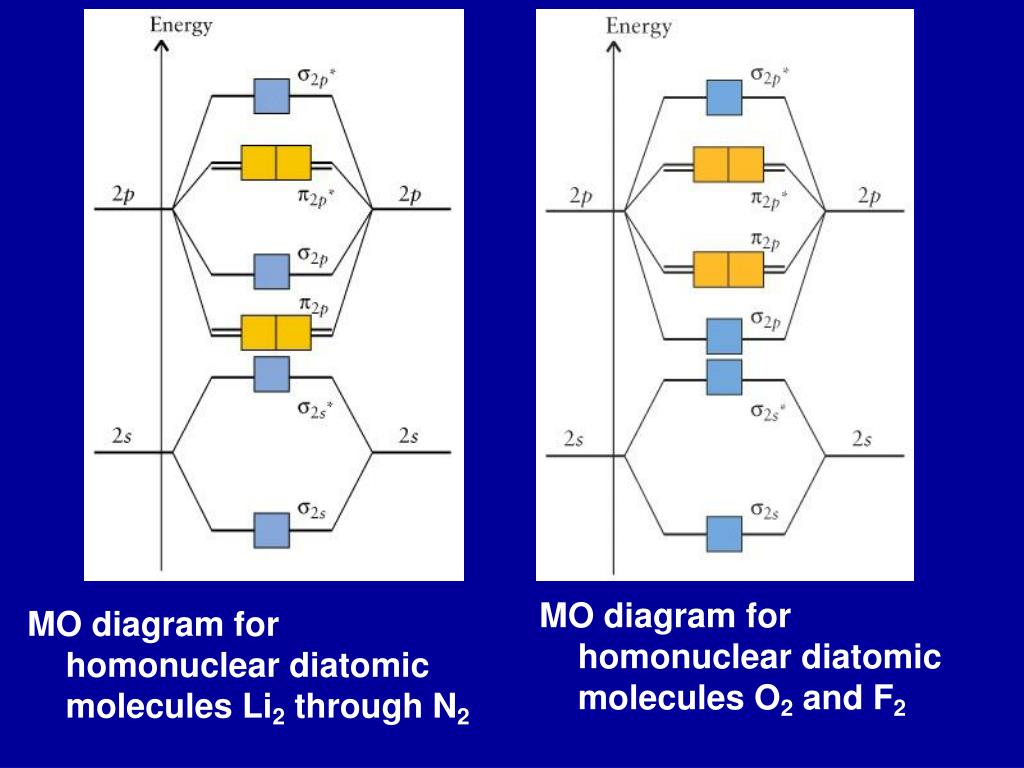
Given below the molecular orbital energy diagram for dinitrogen, N2. It is a relatively inert gas due to the strong triple bond between 2 atoms N. Nitrogen gas can be used to manufacture ammonia NH3, a chemical fertilizer. One of the earliest triumphs of MOT is the discovery of diamagnetic property of N2.
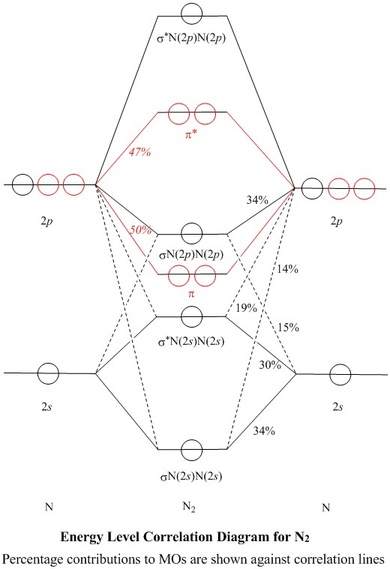
The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.
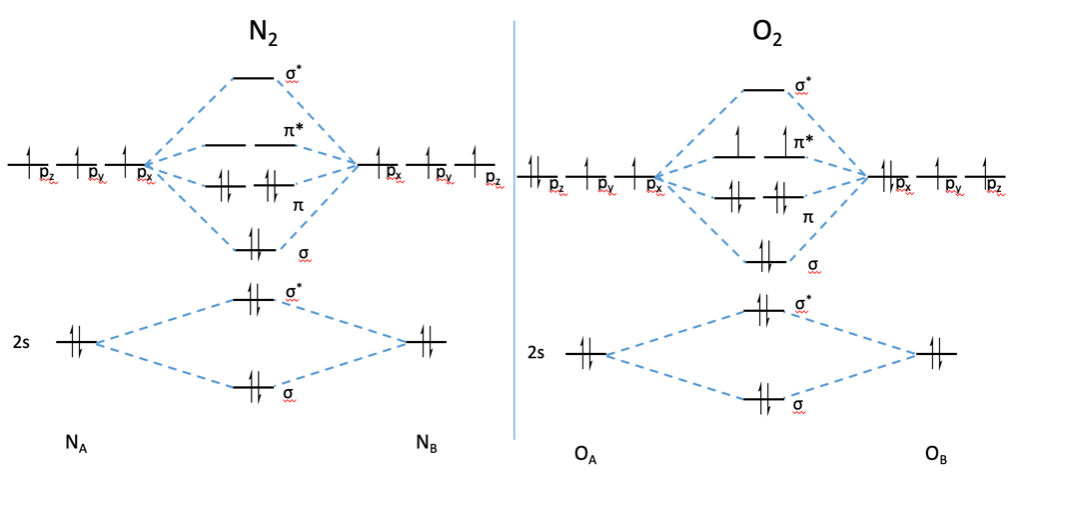
Give The Molecular Orbital Energy Diagram Of N2 And O2 Write The Bond Order Of N2 And O2 Sarthaks Econnect Largest Online Education Community
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable.
N2 is a very stable 10-valence-electron molecule, isoelectronic with CO and with [CN] · The formal bond order of N2 is 3, from about one σ-bond and two π-bonds ...
The molecule can be described as having two pi bonds but without a sigma bond. Dinitrogen[edit]. N2 Molecular Orbital Diagram.
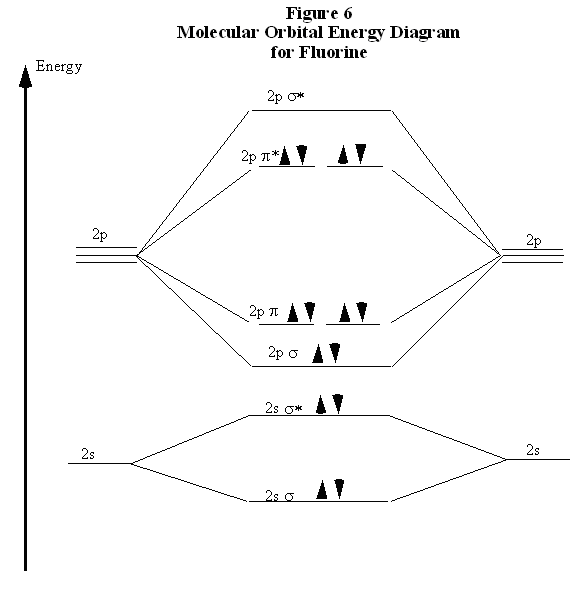
Match. Gravity. Item 1: Part A. By drawing molecular orbital diagrams for B2, C2, N2, O2, and F2, predict which of these homonuclear diatomic molecules are magnetic. By drawing molecular orbital diagrams for , , , , and , predict which of these homonuclear diatomic molecules are magnetic. F2.
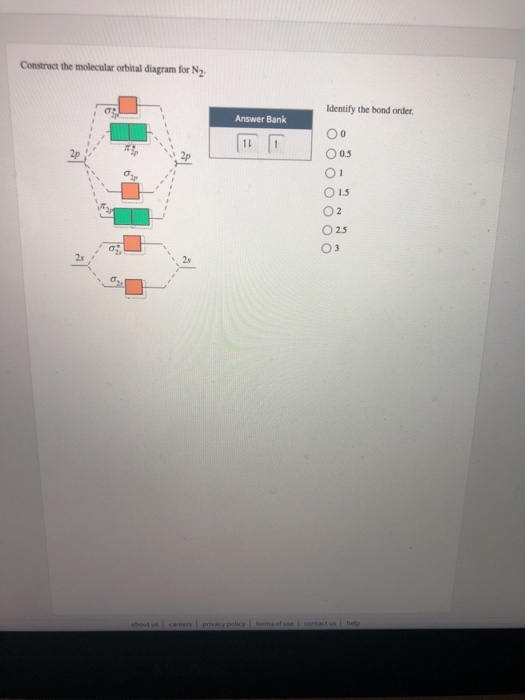
Draw the molecular orbital diagram for n2 ion and calculate the bond order. The molecular orbital diagram of carbon monoxide co is show below. Indicate if it is diamagnetic or paramagnetic. Diatomic molecules made up of two different atoms also have molecular orbital diagrams very similar to that of n2. The other is for after nitrogen starting ...
If we build the MO diagram for "N"_2, it looks like this: First though, notice that the p orbitals are supposed to be degenerate. They weren't drawn that way on this diagram, but they should be. Anyways, for the electron configurations, you would use a notation like the above. g means "gerade", or even symmetry upon inversion, and u means "ungerade", or odd symmetry upon inversion.
Answer (1 of 2): Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma...
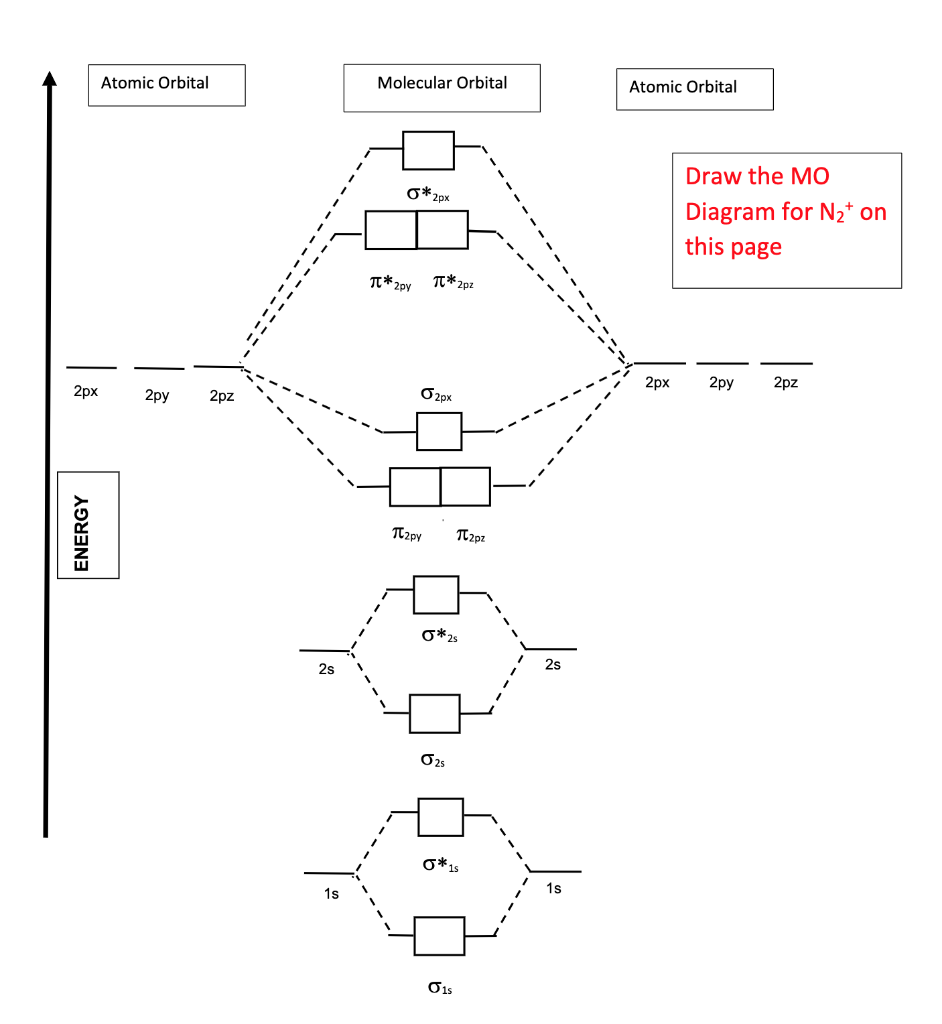
Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Explain What is the relationship between bond order and the dissociation energy of a molecule? ...
Draw the molecular orbital diagram for n2 ion and calculate the bond order. One is for the elements up to nitrogen. The molecular orbital theory mo has been introduced for the diatomic hydrogen molecules. N2 molecular orbital diagram with nitrogen we see the two molecular orbitals mixing and the energy repulsion.
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
N2 molecular orbital energy level diagram also has the following tags. Three filled bonding orbitals. Mo diagrams for diatomic molecules chapter 5 friday october 9 2015. Here is the full molecular orbital diagram for n2. N2 molecular orbital energy level diagram with description. Now we add the 10 electrons 5 from each nitrogen atom. This image ...
Solved Draw The Hybridized Molecular Orbital Picture For Molecular Nitrogen N2 Label Each Orbital E Sp2 Sp3 S And Show How They Overlap In Course Hero
Molecular orbital diagram for n2. Then just fill the. One is for the elements up to nitrogen. For the second period elements the 2s and 2p orbitals are important for mo considerations. Since the s h h orbital shows a decrease in bonding between the two nuclei. Perpendicular to these in the yz plane the 2py orbitals on each atom combine to make ...
Draw the molecular orbital diagram of N2. Also find its bond order and magnetic · Asked by Topperlearning User | 13th Jun, 2016, 02:45: PM. Expert Answer: ...

89 Chemical Bonding 36 Covalent Bonding 35 Molecular Orbital Theory 10 Nitrogen Molecule Madoverchemistry Com
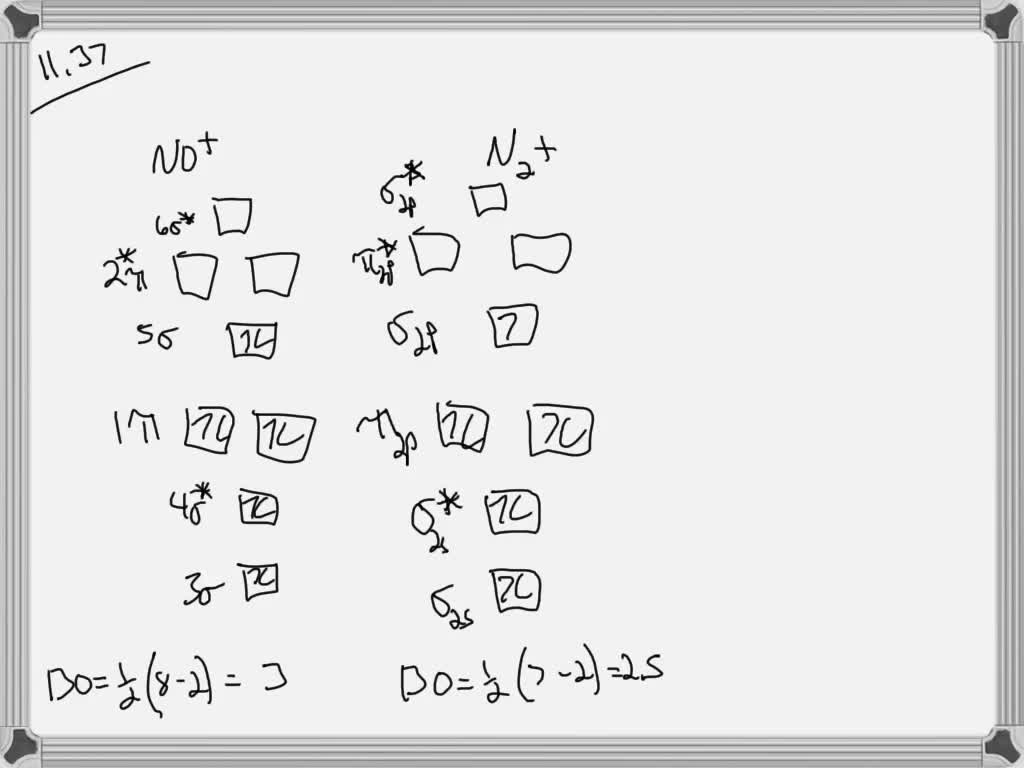
Solved Consider The Molecules No And And N2 Use Molecular Orbital Theory To Answer The Followings A Draw Their Molecular Orbital Diagram Ignore The 1s Electrons B What Is The Bond

Use The Molecular Orbital Energy Level Diagram To Show That N 2 Would Be Expected To Have A Triple Bond F 2 A Single Bond And Ne 2 No Bond




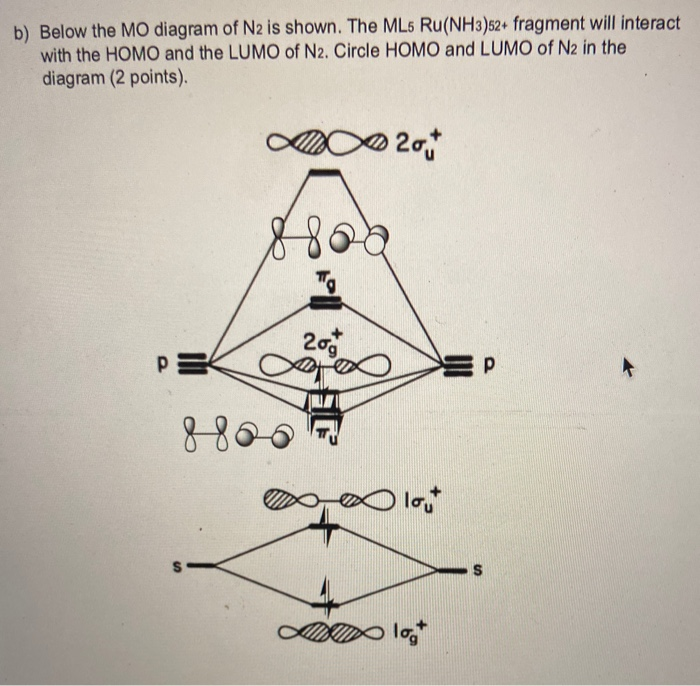












0 Response to "44 mo diagram for n2"
Post a Comment