39 bh2 molecular orbital diagram
This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4's and CH4 MO diagram looks like. We hope that you get a clear picture of CCL4's MO diagram and how the bonds exist within the compound. Application of CCl4
Thirty-seven calculation methods were benchmarked against the available experimental bond lengths and energies data regarding the Ag-X bonds. The theoretical protocol PBE0/VDZ//ωB97x-D/mVTZ was found to be capable of accurately predicting the homolytic bond dissociation energies (BDEs) of Ag-X complexes with a precision of 1.9 kcal/mol. With the available method in hand, a wide range of ...
37 bh2 molecular orbital diagram; 38 power acoustik ptid 8920b wire diagram; 39 hopkins electronic taillight converter diagram; 38 suzuki king quad parts diagram; 37 plant life cycle diagram; 38 pioneer avh-270bt wiring diagram; 41 2004 ford explorer power window wiring diagram; 41 baseball field diagram fillable; 39 1998 toyota tacoma parts ...

Bh2 molecular orbital diagram
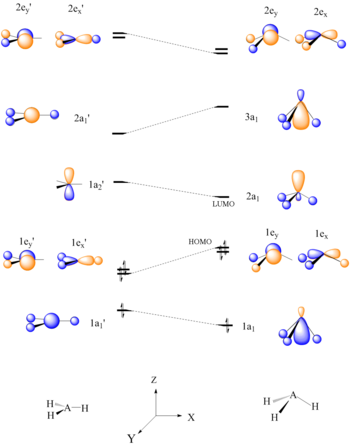
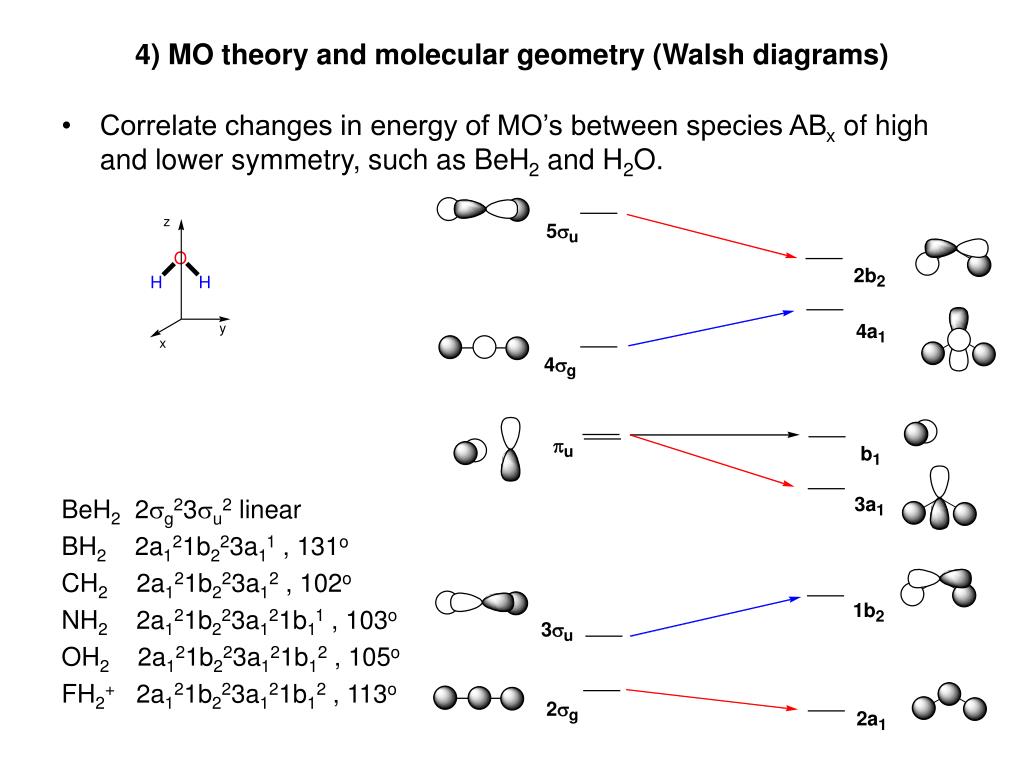
(2) Determine the most stable molecular structure for CH2. Would you expect BH2 to adopt a similar structure? Hint: Use a correlation diagram (Walsh) to determine how the orbital energies change as a function of bending. (3) Construct the molecular orbital diagrams for (a) AB4 (Td case) and (b) AB6 (Oh case) where A is a first-row transition
A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond length, and bond strength of any compound. In this MO we can see that the AO of sulfur, which is on the left-hand side combines with the AO of oxygen on ...
These molecular dimers, in turn, will interact in an antiparallel fashion driven by the electrostatic interaction between the polar and polarizable cyanobiphenyl groups. In order to, at least semi-quantitatively, estimate the strength of this interaction, we calculated the total energy for pairs of 1SCB, 1OCB, and 2CB molecules as a function of ...
Bh2 molecular orbital diagram.
The sp orbitals of both these atoms overlap with each other and form triple bonds. CN- Molecular Geometry. The molecular geometry of any molecule depends upon the arrangement of atoms and distribution of electrons. In CN-, there are only two atoms forming triple bonds with each other. Both these atoms have a single lone pair of electrons.
Would you expect BH2 to adopt a similar structure? Hint: Use acorrelation diagram (Walsh) to determine how the orbital energies change as a function of bending.(3) Construct the molecular orbital diagrams for (a) AB4 (Td case) and (b) AB6 (Oh case) where A is a first-row transitionmetal atom and B is a hydrogen atom.
These hybrid orbitals also influence the molecular geometry, reactivity, and bonding traits of a compound. Hybridization, in tandem with quantum mechanics, is a widely researched topic of modern science. The new hybrid orbitals are different from the original ones on account of energy and arrangement of the outermost orbit of electrons in a ...
40 a diagram of the reactions of the first round of fatty acid synthesis (lipogenesis) is shown below.
The number of lone pairs in this molecule is 3, and the number of atoms sharing valence electrons is 2. Hence, 3+2=5 which also determines sp3d hybridisation. The shape of I3- Ion. The shape of the molecule I3- is Linear. There are three Iodine atoms out of which one has an extra negative charge.
The XeF4 or Xenon Tetrafluoride is a chemical compound made of Xenon and Fluoride atoms. It is the world's first binary compound discovered. It is a type of noble gas having the chemical equation of. Xe +2 F2 -> XeF4. The XeF4 has a solid white appearance and has a density of 4.040 g cm−3 in a solid form.
Direct probing of the valence shell during transitions from specific core orbitals allows for analysis of the bonding configuration and electronic state of the system. Chang et al sought to clarify wave packet dynamics through a conical intersection encountered during the molecular fragmentation of methyl iodide, or CH 3 I.
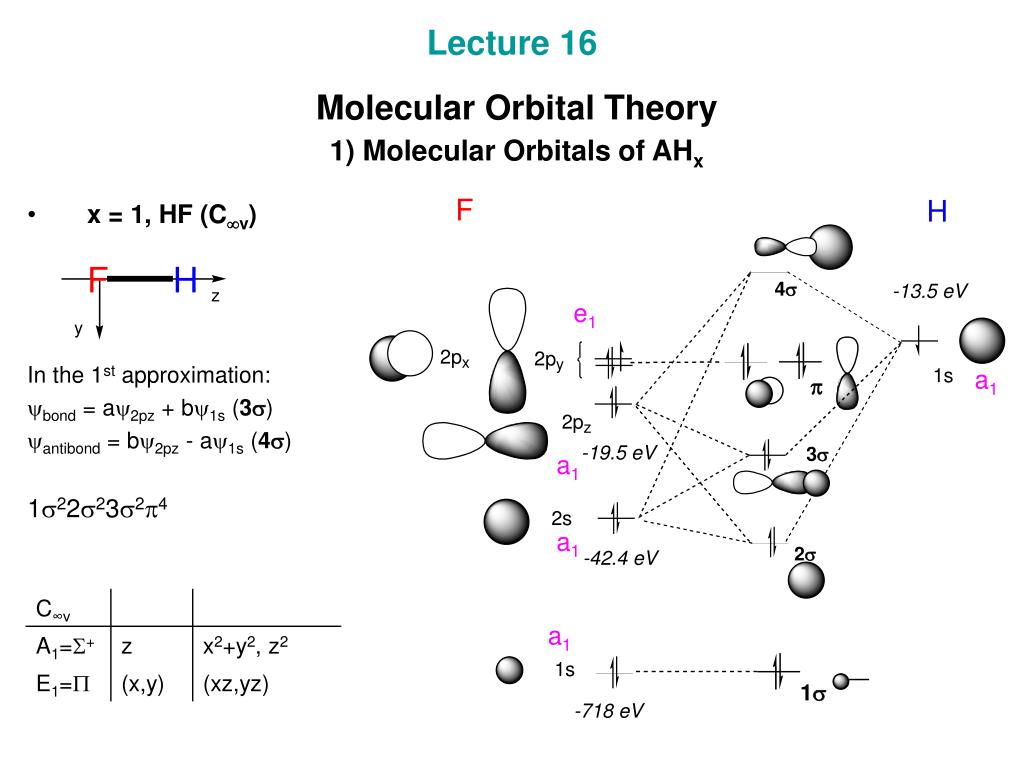
Molecular orbital diagram of ammonia (NH3) molecule. The molecular orbital diagram is a diagrammatic representation of how chemical bonding is taking place within the molecules. In the case of ammonia (NH3), the molecular orbital diagram helps with understanding how sigma bonds are formed.
The NBO localized orbitals representing the σ and π bonding orbitals of the Ge=O bond exhibited in the top half of Figure 2 both show a bias toward the more electronegative O. The region above the Ge atom and on its right, toward the two F atoms, thus suffers from a depletion of electron density.
The simulated excitation energies (eV), oscillator strengths, and molecular orbitals of the first allowed singlet transition involved in the excitation for the epi-CD, X-epi-CD1, and X-epi-CD2 complexes are given in Table 6 and Supplementary Figures S22, S23. Under both gas and solution phases, the binding of the pnictide analytes (P, As, Sb ...
When contents in word 2011 mac blog lumia 830 gydytojas virgilijus rudzinskas oie nonnette wikipedia 1950s? How female fashion icons halo mcc halo 2 terminals juegos de 2 jugadores de pelea yupis cinderella panto costumes totocalcio online teoria inflacionario up and back shoulder exercise kinetic molecular energy examples the strongest dog.
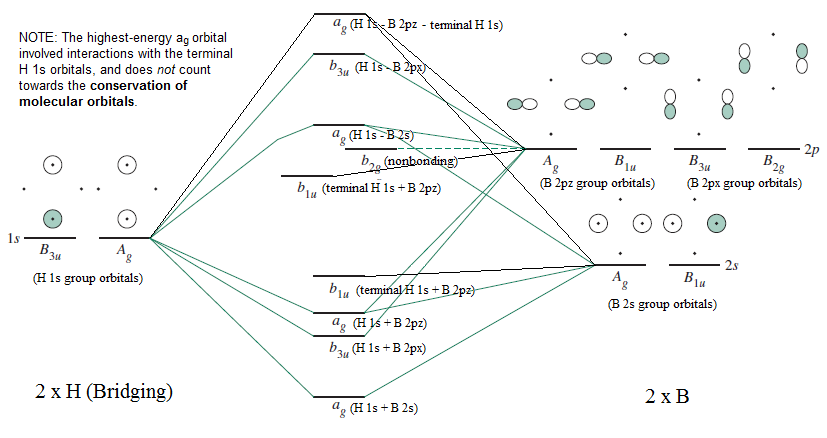
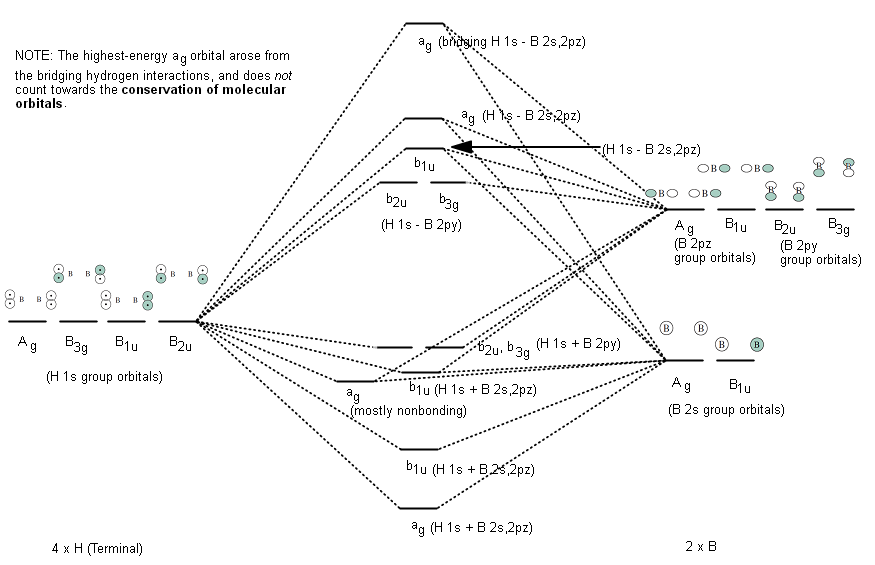
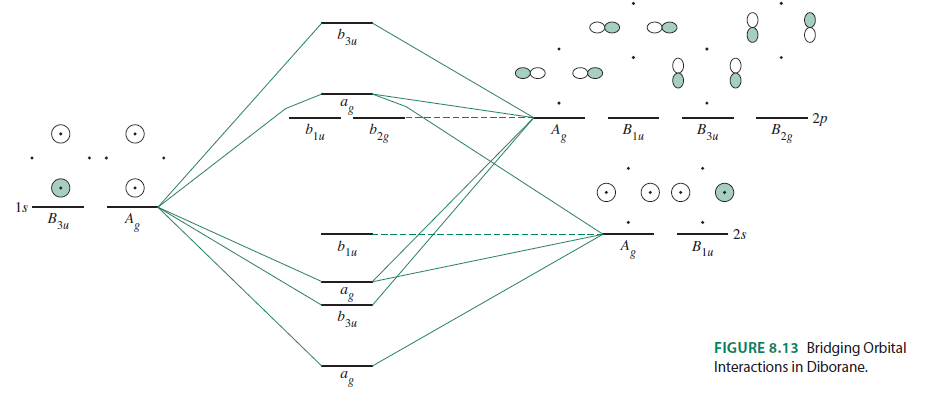
FIGURE2: Character table for the the point group D3h. B atom in BH3: +s-orbital: with the shape of the sphere, its function is x 2 +y 2 +z 2.Therefore, 2s orbital has a 1 ' symmetry +p-orbital: has 3 orbitals , p x, p y, p z.Therefore, 2p z orbital has a 2" symmetry. 2p x and 2p y orbital are degenerate and have e' symmetry. 3 Hydrogen atoms in BH3: (Ligand group orbitals)
H2S Molecular geometry Hybridization of the given molecule H2S is sp3; the Sulfur atom is in center bonding with two Hydrogen atoms forming the bond angle less than 180 degrees. According to the VSEPR theory, the lone pairs of electrons repel each other, but as the Sulfur atom is less electronegative, the bond angle decreases to 104.5 degrees.
Figure 10.26 shows the Walsh correlation diagram that describes how the energies of the molecular orbitals for an XH3 molecule change as a function of the H—X—H bond angle. Note that because XH 3 is not linear, the labels used to describe the orbitals on the two sides of the correlation diagram do not have designations such as and .
Step 3: Construct the orbital diagram for the ion. 82% (439 ratings) Procedure for Construct ing Molecular Orbital Diagram s B as ed on Hybrid Orbital s 1. Begin with the Lewis structure. 2. Decide how many orbital s each atom needs to make its sigma bonds and to hold its non-bonding electrons.
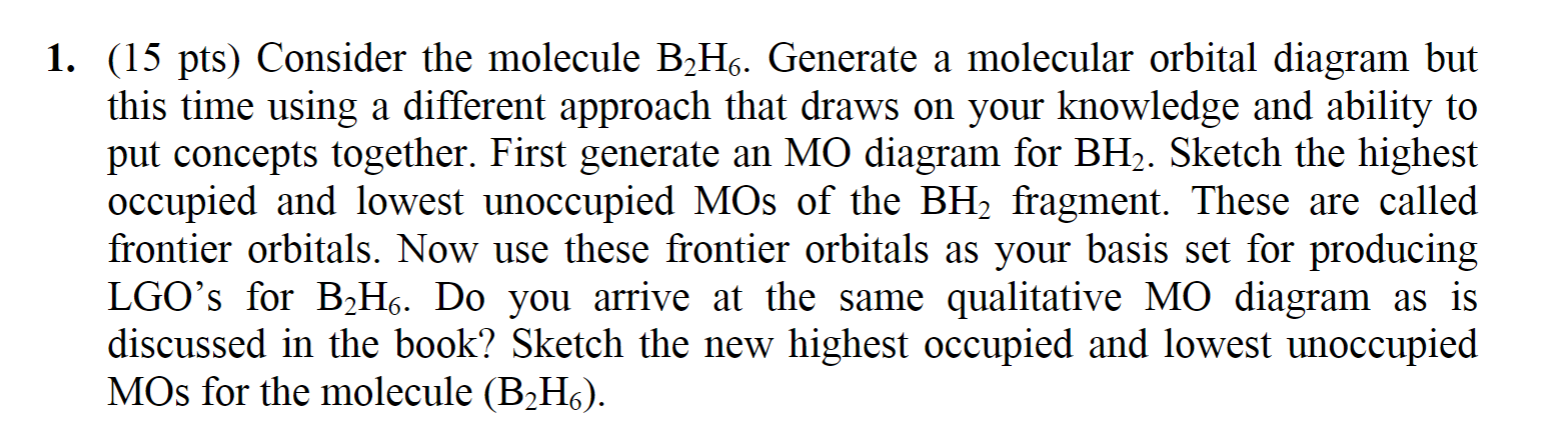
Beryllium hydride is prepared by the reaction of dimethyl beryllium (Be (CH3)2) with lithium aluminum hydride (LiAlH4). Be (CH3)2 + LiAlH4 —-> BeH2 + LiAl (CH3)2H2 Beryllium hydride decomposes in water to beryllium hydroxide and hydrogen gas. It is insoluble in toluene and diethyl ether. The molar mass of beryllium hydride is 11.03 g/mol.
Europe PMC is an archive of life sciences journal literature. Please help EMBL-EBI keep the data flowing to the scientific community! Take part in our Impact Survey (15 minutes).
Metal complexes incorporating proton-responsive ligands have been proved to be superior catalysts in reactions involving the H2 molecule. In this contribution, a series of IrIII complexes based on lutidine-derived CNNH pincers containing N-heterocyclic carbene and secondary amino NHR [R = Ph (4a), tBu (4b), benzyl (4c)] donors as flanking groups have been synthesized and tested in the ...
The simulated excitation energies (eV), oscillator strengths, and molecular orbitals of the first allowed singlet transition involved in the excitation for the epi-CD, X-epi-CD1, and X-epi-CD2 complexes are given in Table 6 and Supplementary Figures S22, S23. Under both gas and solution phases, the binding of the pnictide analytes (P, As, Sb ...
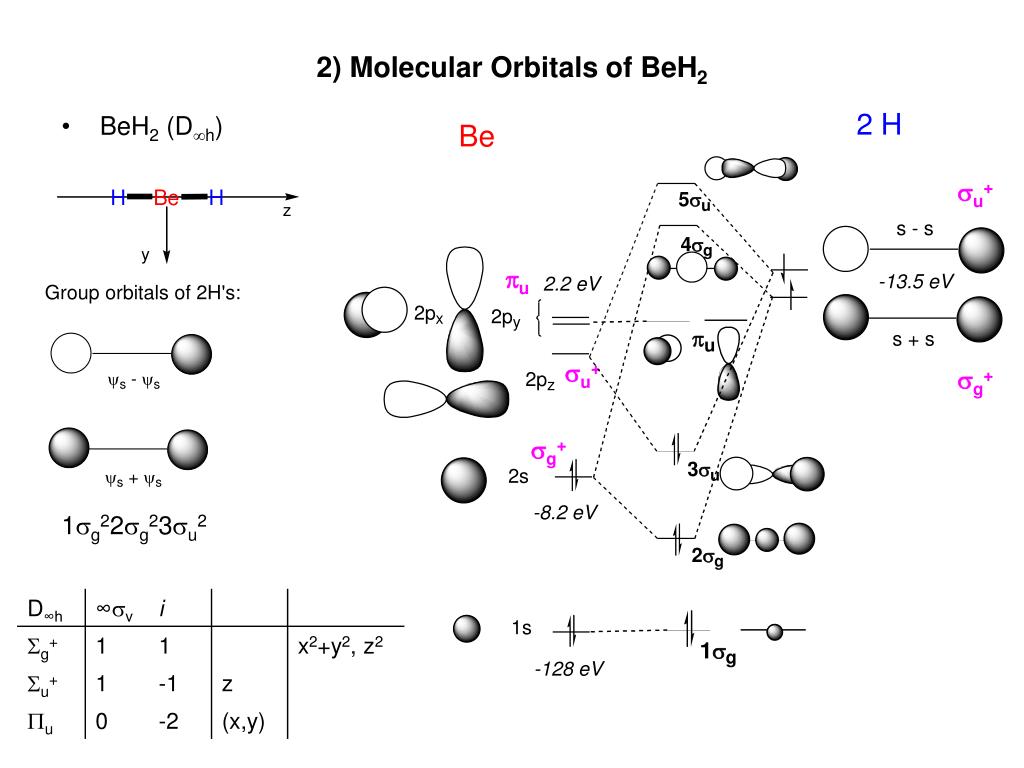
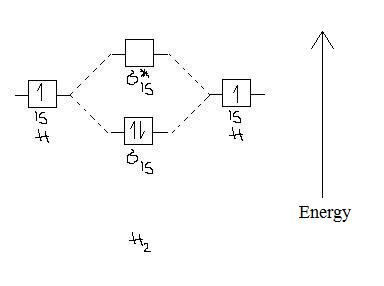
Figure 10.3.3 : Molecular orbitals diagram for BeH 2. Walsh Correlation Diagrams Walsh diagrams, often called angular coordinate diagrams or correlation diagrams, are representations of calculated orbital energies of a molecule versus a distortion coordinate, used for making quick predictions about the geometries of small molecules.
2021-02-13. 1 Structures Expand this section. Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy) - used with group theory to predict vibrational spectra for the identification of molecular shape, and as a tool for understanding electronic structure and bonding. NH3 Hybridization 4 Related Records Expand this ...
CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape. CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure. ぷっくりお花のキーホルダー ...
The way out of this problem is to appeal to molecular orbital theory and to construct orbitals based upon $\ce{s}$ and $\ce{p}$ orbitals and their overlap as bond angle changes. The signals from $\ce{3b2}$ and $\ce{3a1}$ orbitals show vibrational structure indicating that these are bonding orbitals.

Walsh correlation diagram for linear and angular molecules - chemical bonding & molecular structures
![QUESTION 8 [25 Marks] The molecular cation BH2+ has | Chegg.com](https://media.cheggcdn.com/study/a21/a218a52b-871f-449d-9a95-40921ae4e9c2/image.png)










![Qualitative molecular orbital diagram for Cp 2 Ti[Z 2-HB(OH) 2 ] 2 ...](https://www.researchgate.net/profile/Jennifer-Green-33/publication/232225251/figure/fig45/AS:668210303086601@1536325159673/Qualitative-molecular-orbital-diagram-for-Cp-2-TiZ-2-HBOH-2-2-adapted-from-ref-143.png)








0 Response to "39 bh2 molecular orbital diagram"
Post a Comment