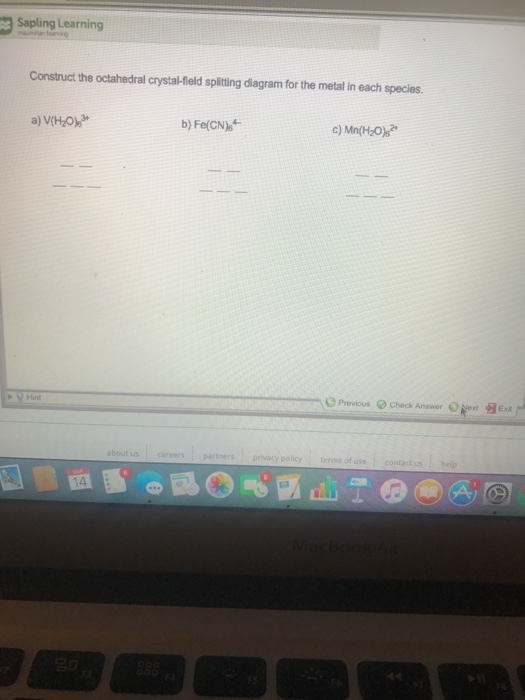
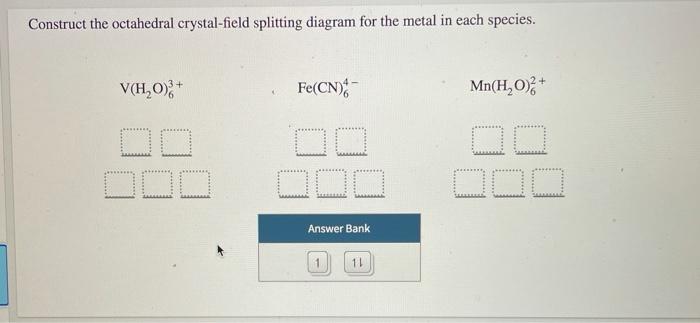
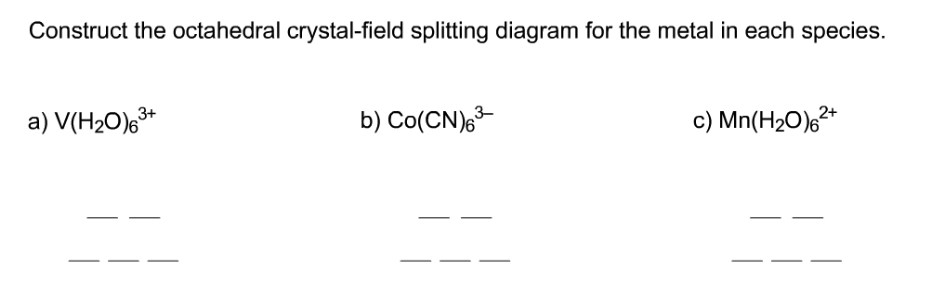
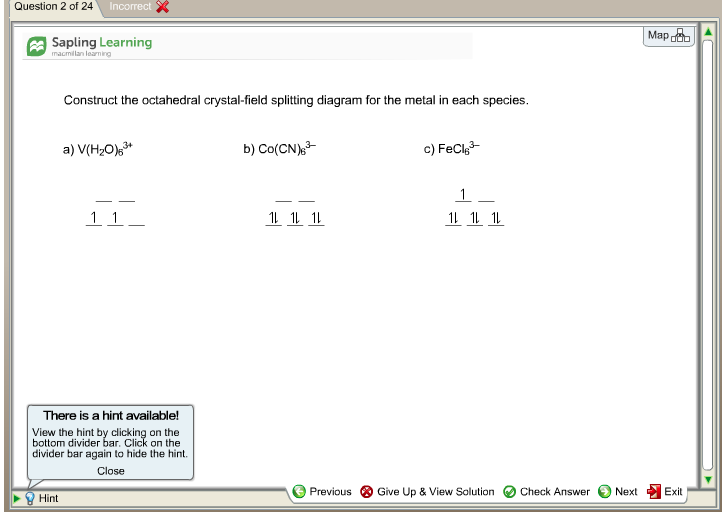
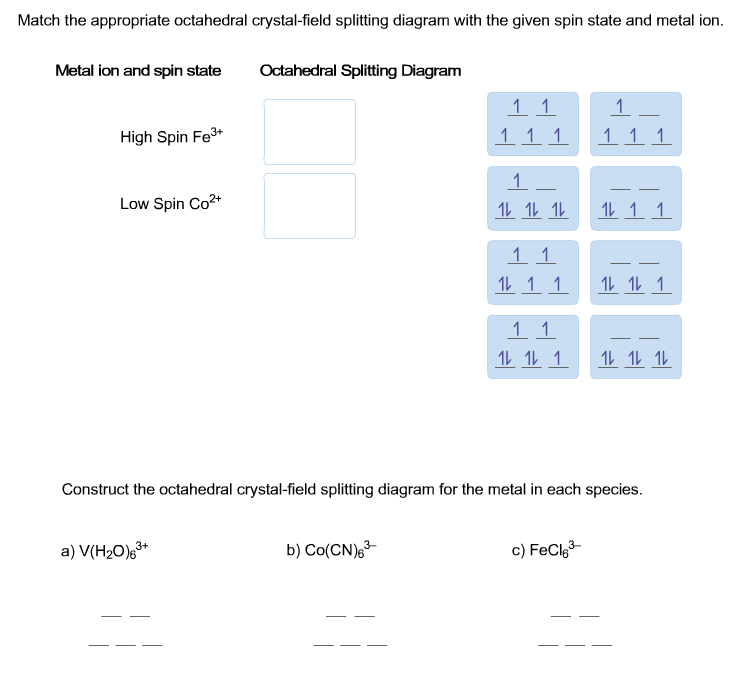
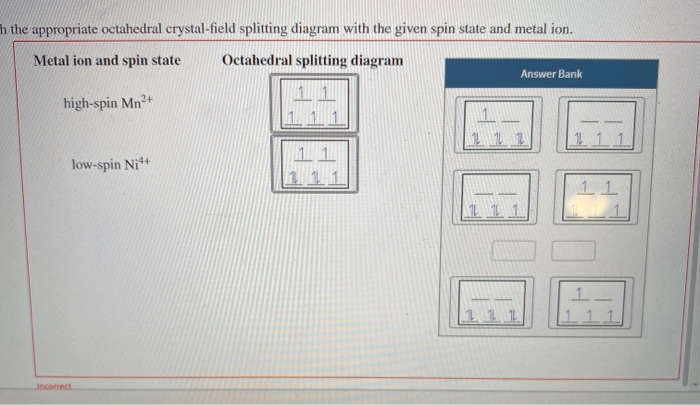
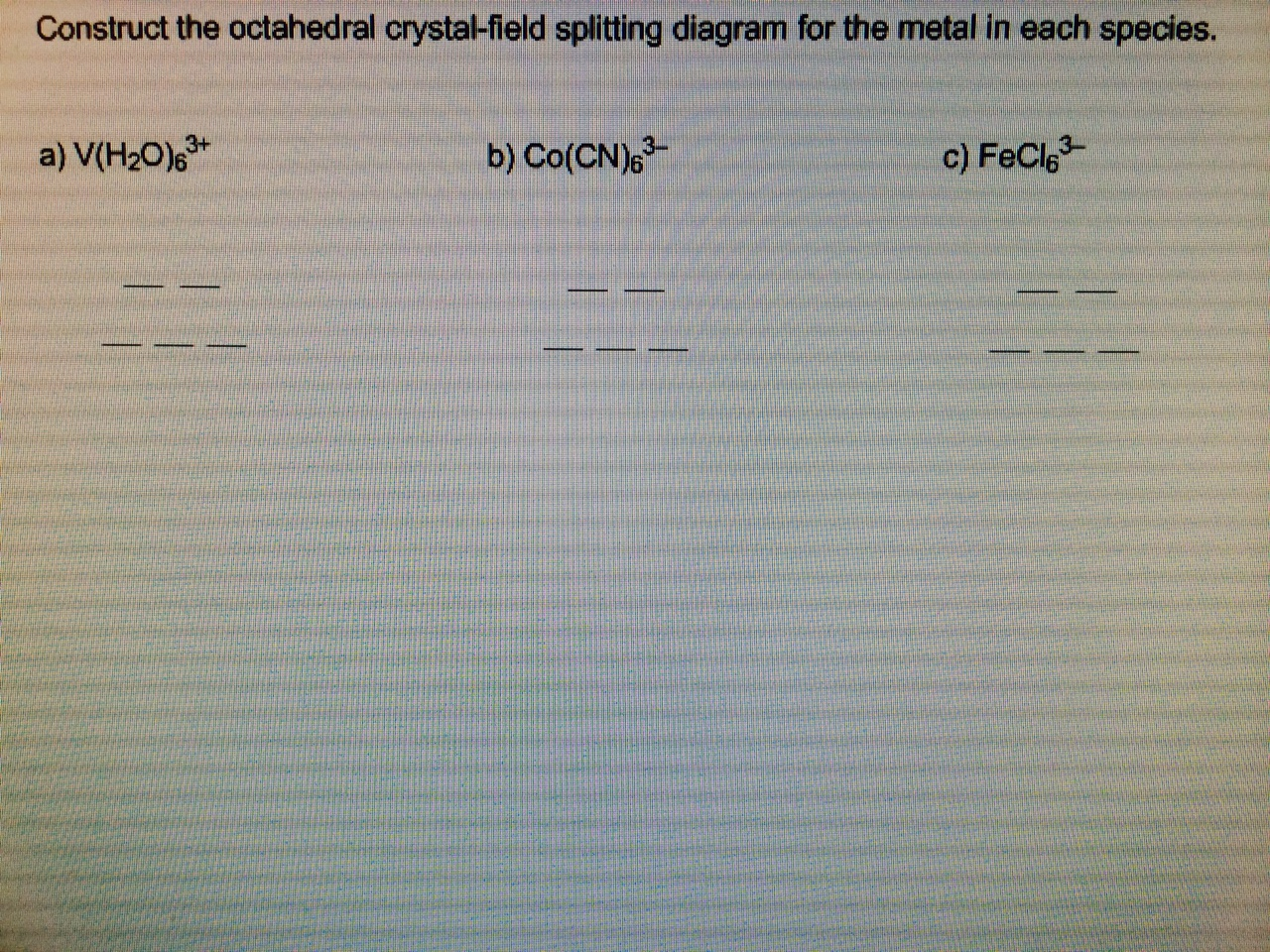
39 construct the octahedral crystal-field splitting diagram for the metal in each species.
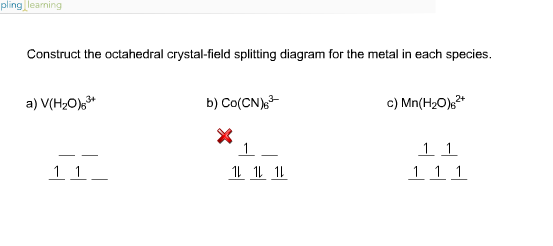
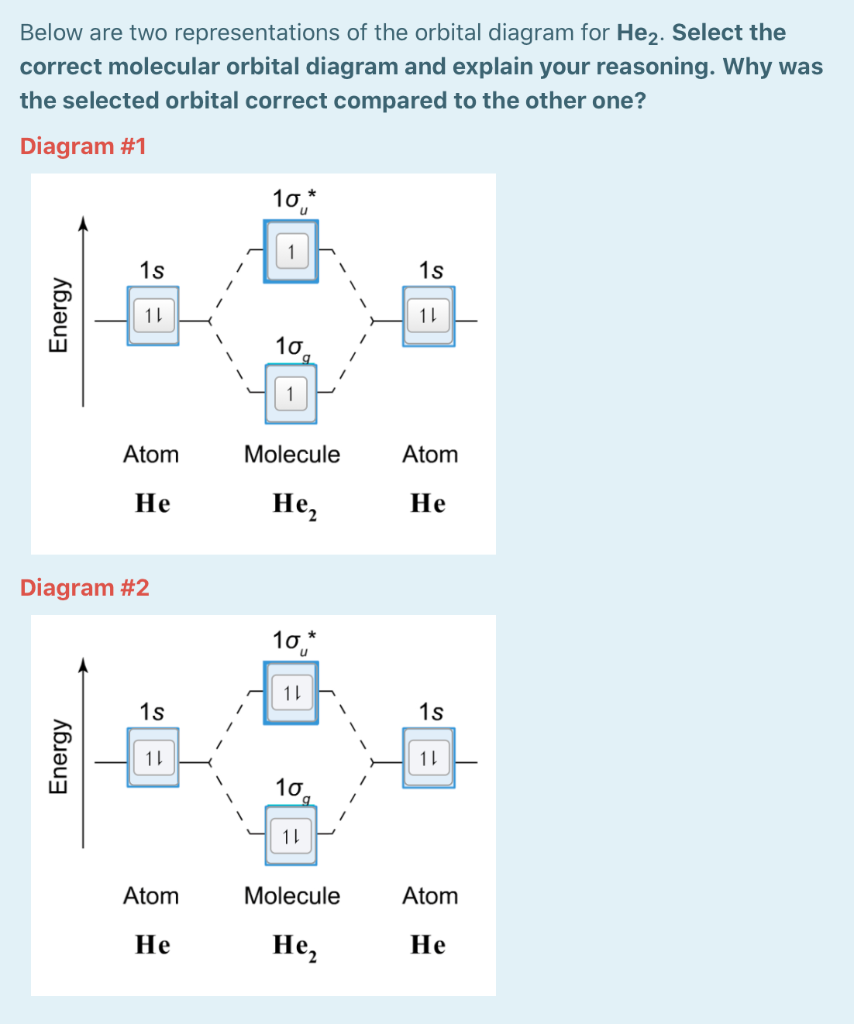
Based on crystal field theory, which of the following metal ions will not be colored when placed in an octahedral crystal field?. Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H2O)63+ Co(CN)63 - Mn(H2O)62+.
Crystal field theory (CFT) describes the breaking of orbital degeneracy in transition metal complexes due to the presence of ligands. Placing a charge of −1 at each vertex of an octahedron causes the d orbitals to split into two groups with different energies: the dx2−y2 and dz2 orbitals increase in energy...
Q: Please help me with this question, thanks. Construct the octahedral crystal-field splitting diagram for the metal in eac.
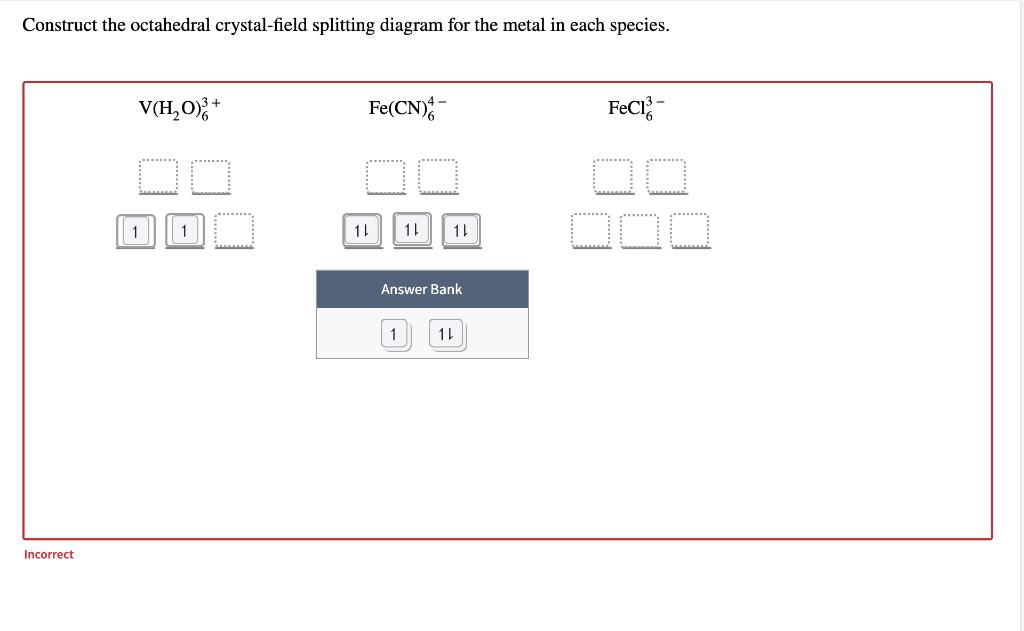
Construct the octahedral crystal-field splitting diagram for the metal in each species.
Trick for Crystal field theory (CFT) of Octahedral & Tetrahedral complexes | Coordination Compounds. Crystal field theory: Splitting of orbitals in Octahedral complexes.
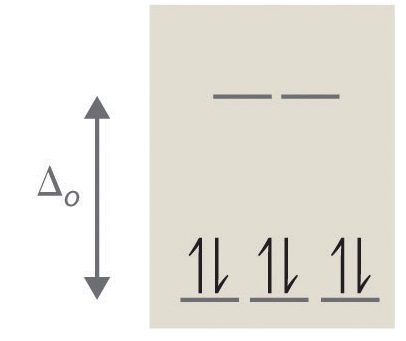
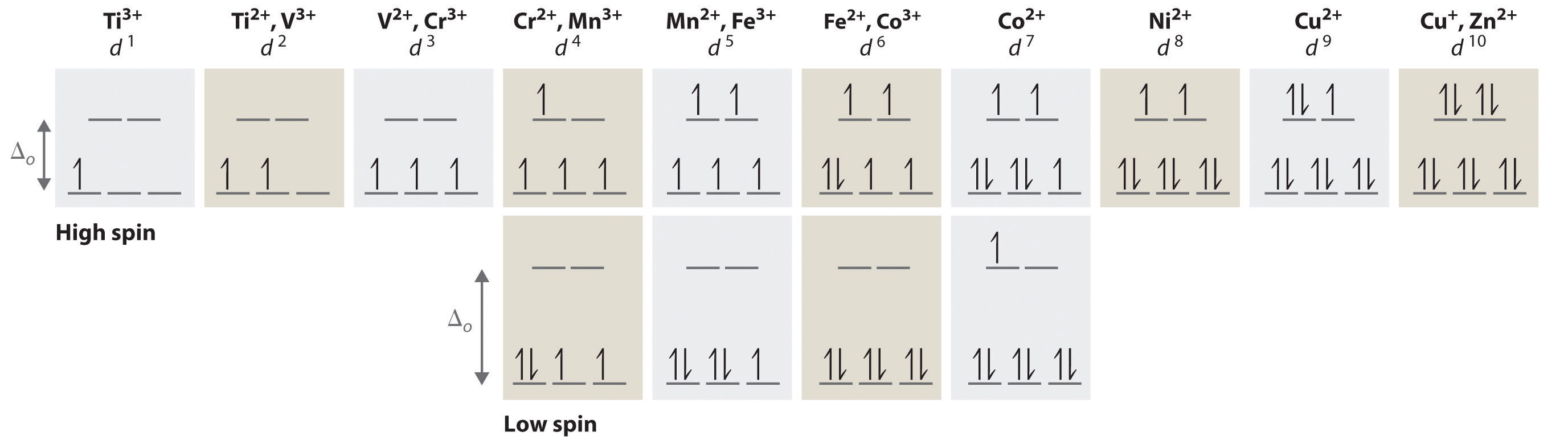
Crystal field d orbital splitting diagrams for common geometries. A splitting of magnitude Δ7 is produced and it depends on both the nature of the metal ion and of the ligand. In the case of the octahedral field each electron placed in one of the t2g orbitals is stabilised by a total of 2/5Δ, while...
Calculate the crystal field splitting energy (in kJ/mol) for this ion. Write the chemical reaction of lithium metal in a solution of silver nitrate. Indicate the charge of each species. Clearly indicate which metal is undergoing oxidation and which metal is undergoing reduction.
Construct the octahedral crystal-field splitting diagram for the metal in each species..
Species coefficient is 1 then 1 needs to be entered in the field before that species. Thanks for visiting this page on this web site we spe...
Q: Balance each of the following equations. Part BHgO(s)→Hg(l)+O2(g)Express your answer as a chemical e... A: A balanced chemical equation means when reactant and products contain same type and number of atoms ...
Lecture 7 crystal field theory for octahedral complexes boats and propellersif you have a single engine. The octahedral splitting energy is the energy Vh2o63 cocn63 mnh2o62 show transcribed image text construct the octahedral crystal field splitting diagram for the metal in each species.
Start studying Crystal Field Splitting Diagram. Learn vocabulary, terms and more with flashcards, games and other study tools. Terms in this set (5). Crystal Field Splitting Octahedral.
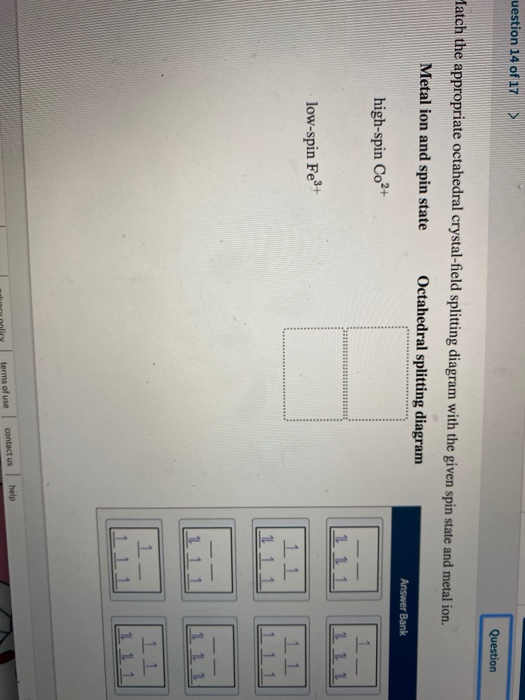
Draw the octahedral crystal field splitting diagram for each metal ion. There is a large energy separation between the d z² orbital and the d xz and d yz orbitals meaning that the crystal field splitting energy is large. We find that the square planar complexes have the greatest crystal field...
Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H2O)63+ Co(CN)63 - Mn(H2O)62+.
If you can't find your institution, please check your spelling and do not use abbreviations. If your institution is not listed, please visit our Digital Product Support Community. Construct the octahedral crystal-field splitting diagram for the metal in each species.
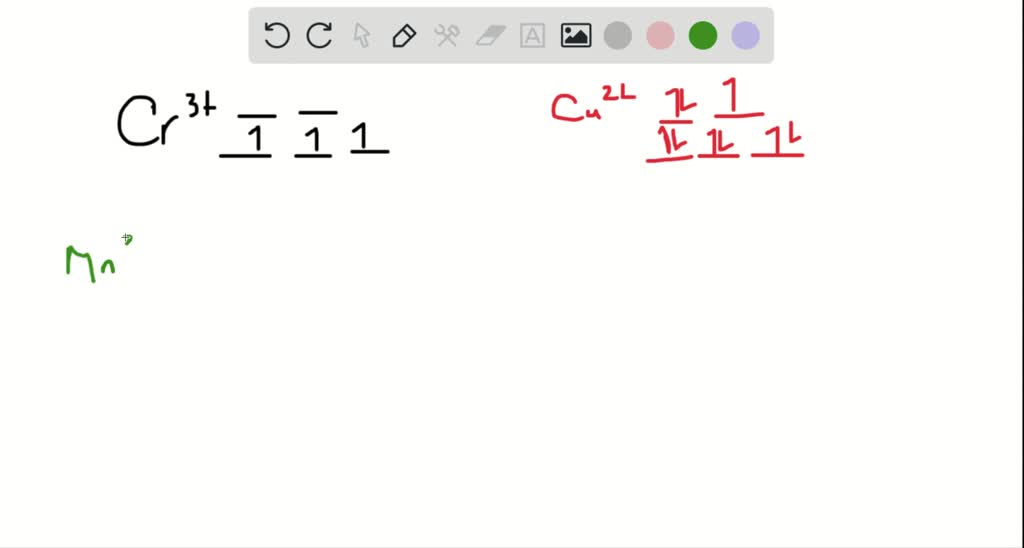
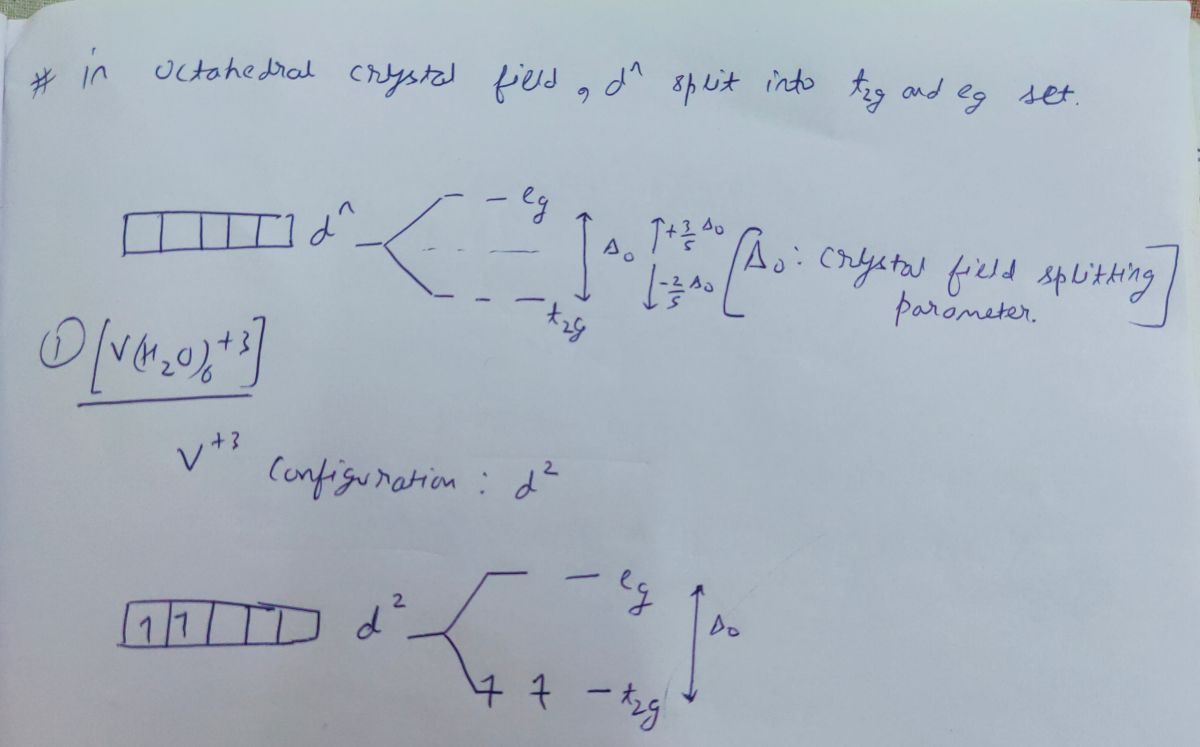
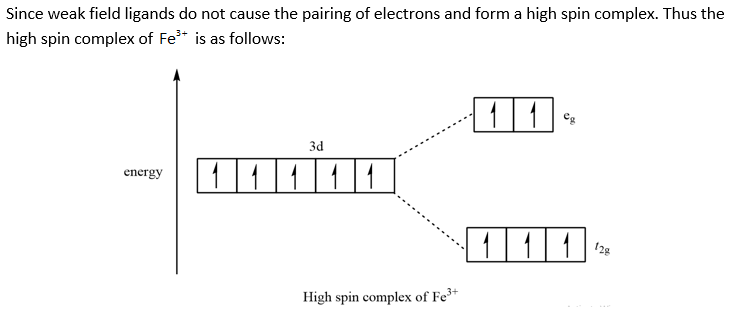
So the octahedron from crystal field splitting takes place like this two off. The orbital's going higher and adjusted, and in the ground state, and three of And this as each your vittles. So for chromium three plus, we have for chromium. We know the chromium silica reconfiguration is like this are going...
The crystal field -splitting for Cr^(3+) ion in octahedral field changes for ligands I^(-),H_(2)O,NH... Among the octahedral and tetrahedral crystal fields, In which case the magnitude of crystal field sp... Explain the following terms giving suitable examples in each case (i) Ambident ligand (ii) Dentici...
If the octahedral crystal field splitting energy (Δo) is greater than the pairing energy (P), it is When the white light falls on the complex ion, the central metal ion absorbs visible light corresponding to the crystal filed splitting energy and transmits rest of the light which is responsible for the colour of the...
The use of the crystal field splitting parameter would seem to be a more sensible parameter to use Figure 1-12. Simplified molecular orbital diagram for the formation of an octahedral ML6 complex in Comparisons with CFSE data for each transition metal ion in octahedral sites in periclase, MgO...
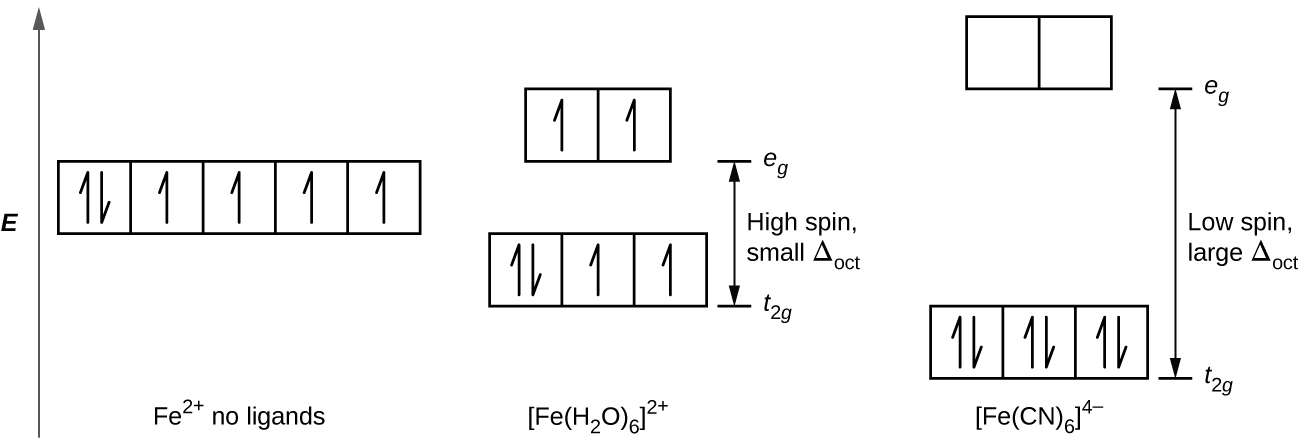
Octahedral Crystal Fields. Each Mn2+ ion in manganese(II) oxide is surrounded by six O2- ions arranged toward the corners of an octahedron, as The magnitude of the splitting of the t2g and eg orbitals changes from one octahedral complex to another. It depends on the identity of the metal ion...
3 for each species molecule or ion in the net ionic equation assign oxidation. Au fe3 ag au3 nb3 Based on crystal field theory which of the following metal ions will not be colored when placed in an It then asks how many unpaired electrons and asks to draw a crystal field splitting diagram for this...
Crystal field splitting for octahedral complex is: So, because of above reason in dx2-y2 and dz2 there is repulsion between the orbitals and ligands In the crystal field theory, we talk about the splitting of degenerate orbitals of Transition elements which happens when the ligands approach the metal atom...
So there are special properties of coordination complexes, so that's where you have a transition metal in the middle This difference is called the octahedral field splitting energy, because OK, so that's a crystal field splitting diagram for an octahedral case, and now let's look at some examples of this.
Octahedral CFT splitting: Electron diagram for octahedral d shell splitting. Therefore, the crystal field splitting diagram for square planar geometry can be derived from the octahedral diagram. For example, monomeric Ti(III) species have one d electron and must be (para)magnetic, regardless...
octahedral crystal field splitting for a d metal ion 5 d-orbitals. Crystal Field Stabilization Energies for the Octahedral Geometry Weak Field dn Configuration CFSE d1 t2g1 eg0 Jahn-Teller Effect The Jahn-Teller theorem states that any species with an electronically degenerate ground state will distort...
Crystal field theory octahedral geometry for coordination compounds. Based on crystal field theory which of the following metal ions... A d1 octahedral complex is found to absorb visible light with the absorption maximum occcurring at 523 nm. 3 for each species molecule or ion in the net ionic...
For transition metal cations that contain varying numbers of d electrons in orbitals that are NOT spherically That is, the exact opposite of the situation we just dealt with for the octahedral crystal field. Note that a different CFT energy splitting diagram has to be applied for each stereochemistry.
Crystal field theory (CFT) describes the breaking of degeneracies of electron orbital states, usually d or f orbitals, due to a static electric field produced by a surrounding charge distribution (anion neighbors).
The crystal field splitting is based on where the ligands (modelled as point charges) are in relation to the orbitals. In an octahedral complex, the ligands are all at 90° from each other and are placed on each of the $x,$ $y,$ $z$ axes. The orbitals that lie on these axes will experience the most repulsion...
The crystal field theory (CFT) is an electrostatic model which considers the metal-ligand bond to be ionic arising purely from electrostatic interactions between the metal ion Crystal field for octahedral complexes. In an octahedral complex, there are six ligands attached to the central transition metal.
...the octahedral crystal-field splitting diagram for the metal in each species. a) v(h2o)63 › construct the octahedral crystal-field splitting With crystal field theory. If you draw the oribital splitting diagram you will find that there are different electronic transitions ie. Fe in the compound is...
Table 23.10 Crystal Field Splitting Energies for Some Octahedral (Δo)* and Tetrahedral (Δt) Transition-Metal Complexes. CFSEs are important for two reasons. First, the existence of CFSE nicely accounts for the difference between experimentally measured values for bond energies in...
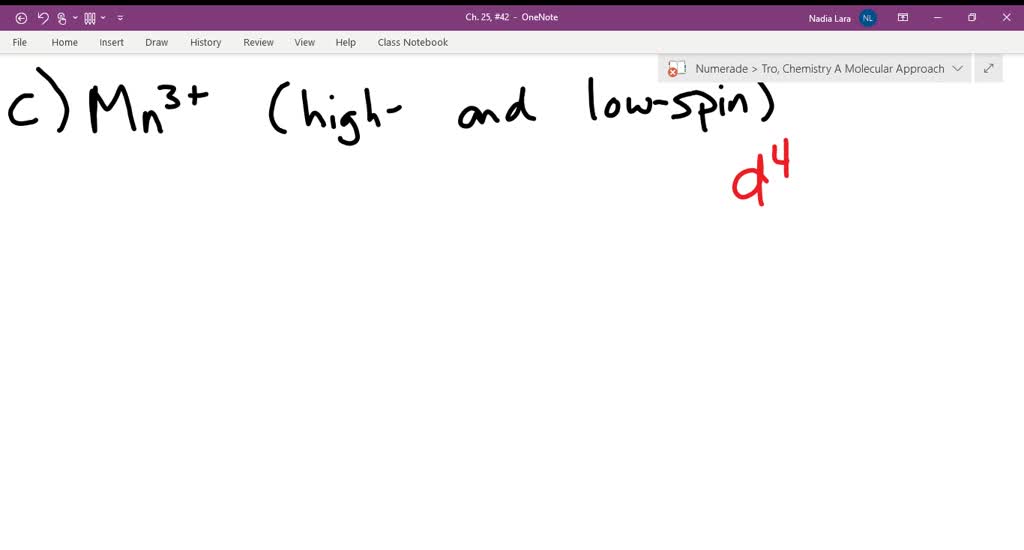
Construct the octahedral crystal field splitting diagram for the metal in each species you are currently in a labeling module turn off browse mode or quick nav tab to items space or enter to 19623
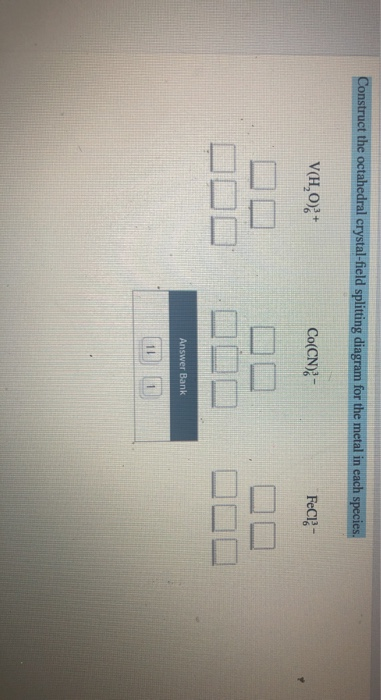
Construct the octahedral crystal field splitting diagram for the metal in each species vh08 fecns feci3 answer bank 22681
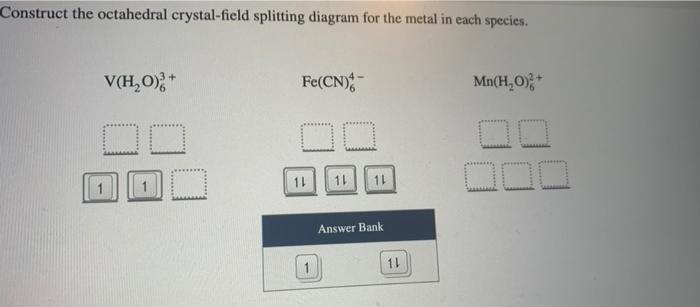
Question 9 of 12 construct the octahedral crystal field splitting diagram for the metal in each species_ vo cocn mnh0 answer bank 43684













0 Response to "39 construct the octahedral crystal-field splitting diagram for the metal in each species."
Post a Comment