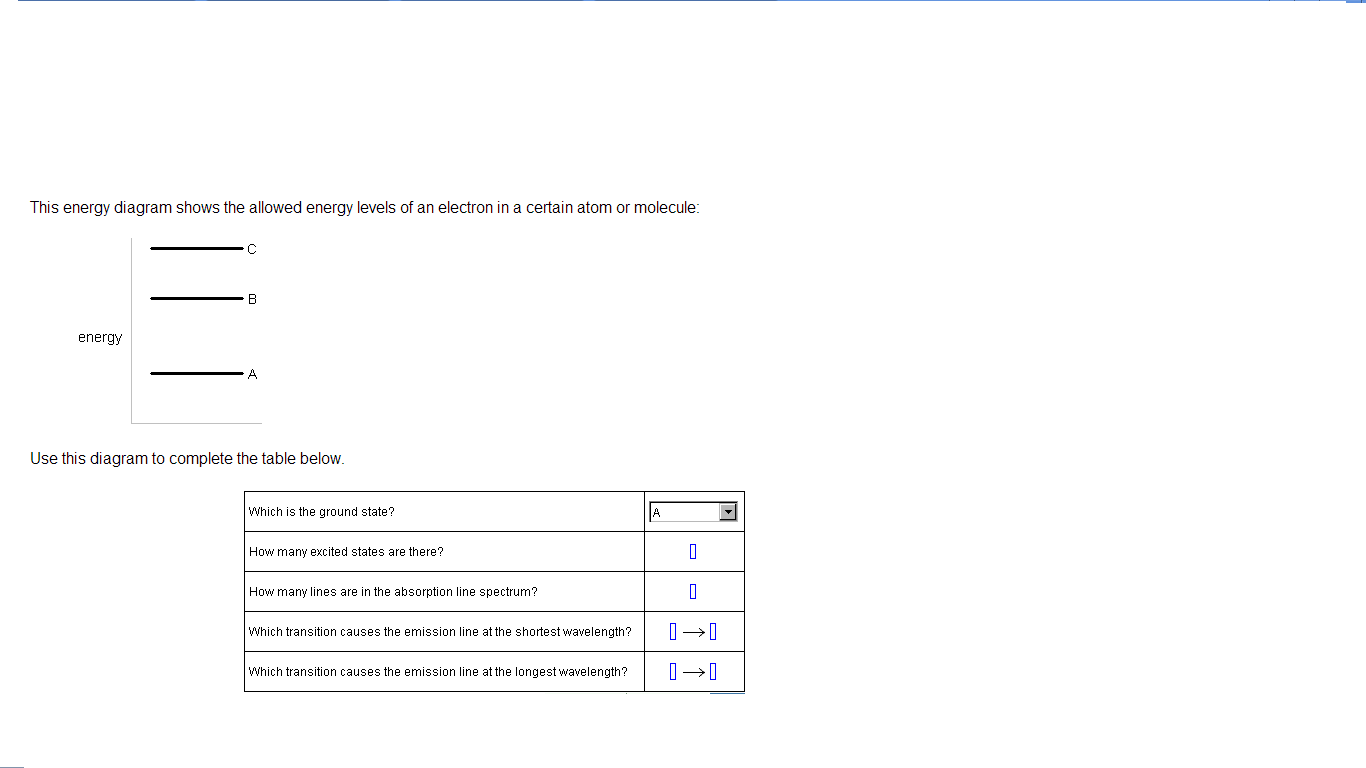
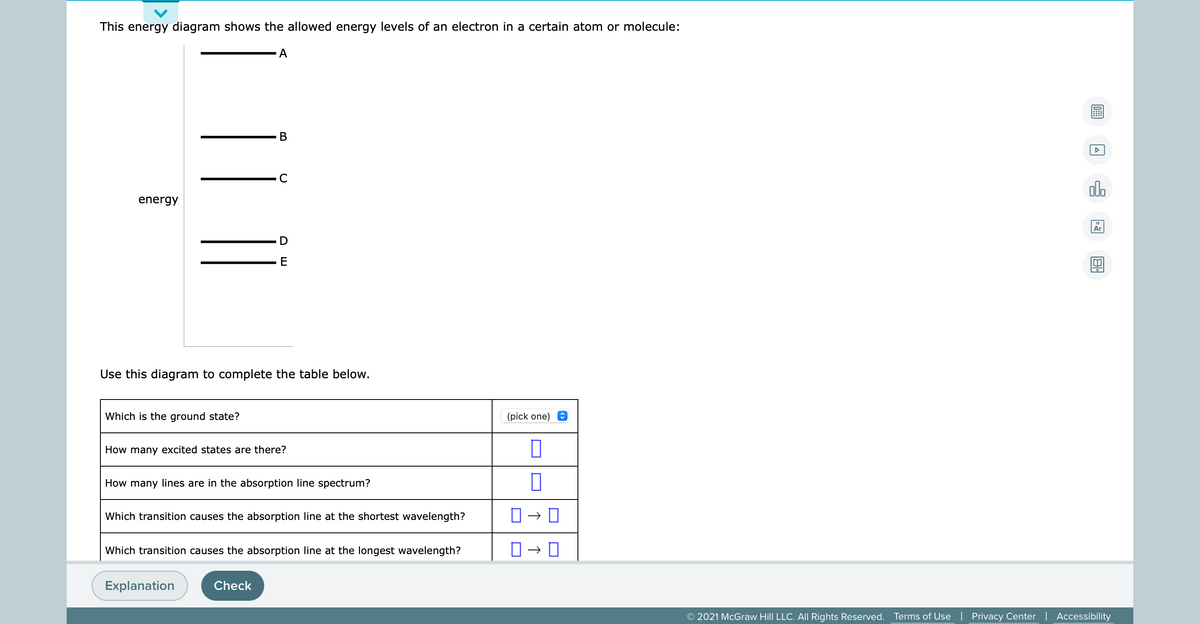
40 this energy diagram shows the allowed energy levels of an electron in a certain atom or molecule:
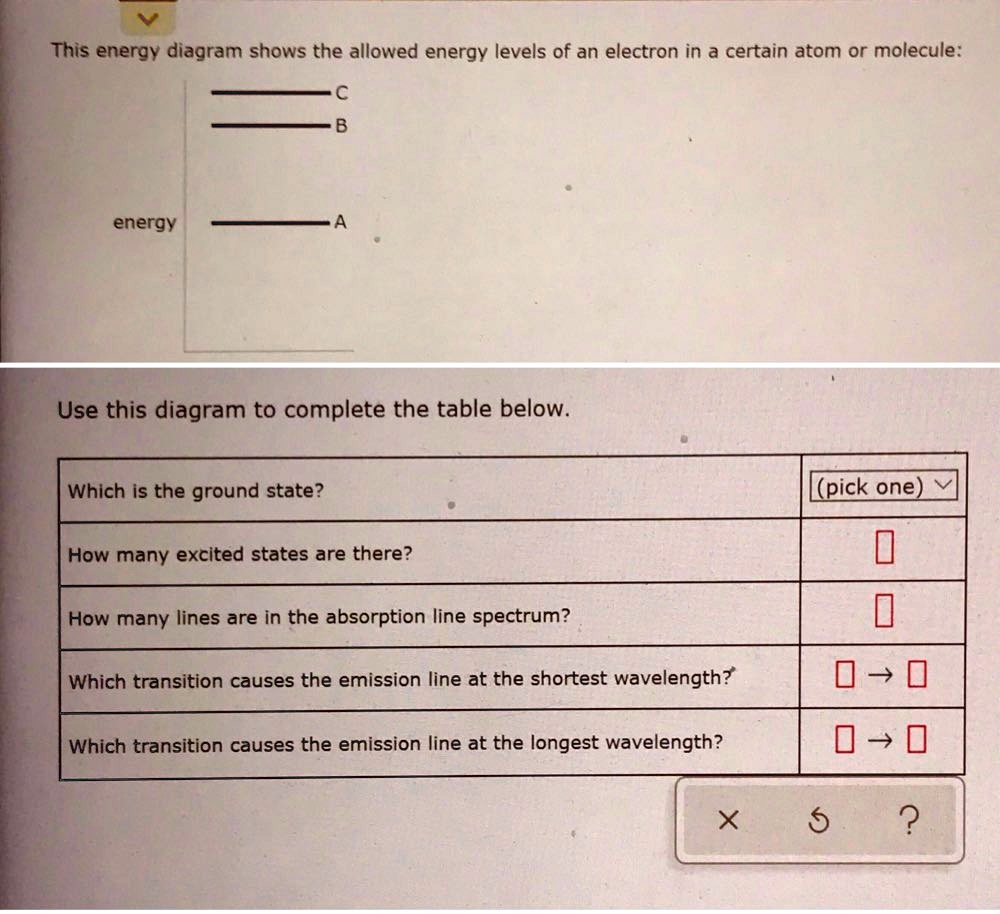
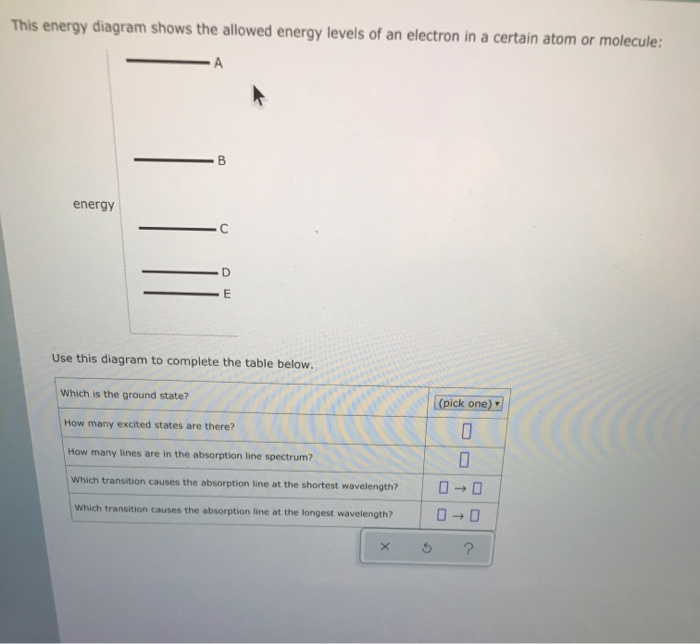
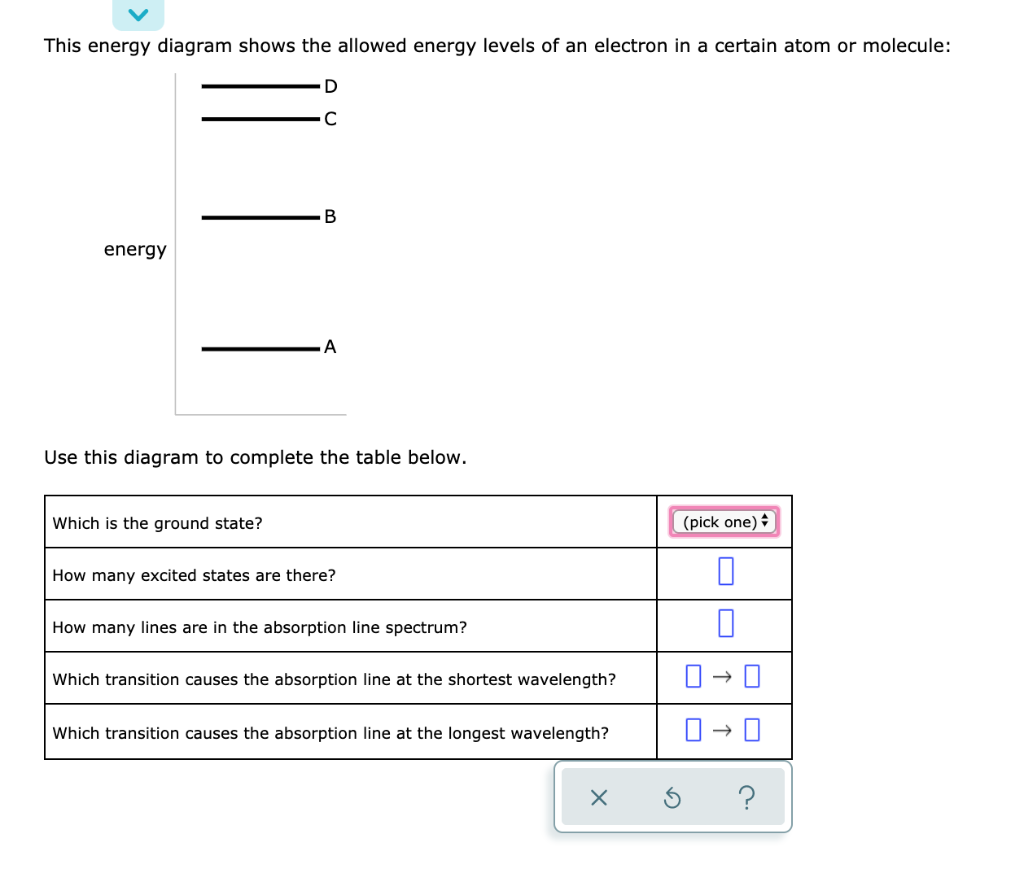
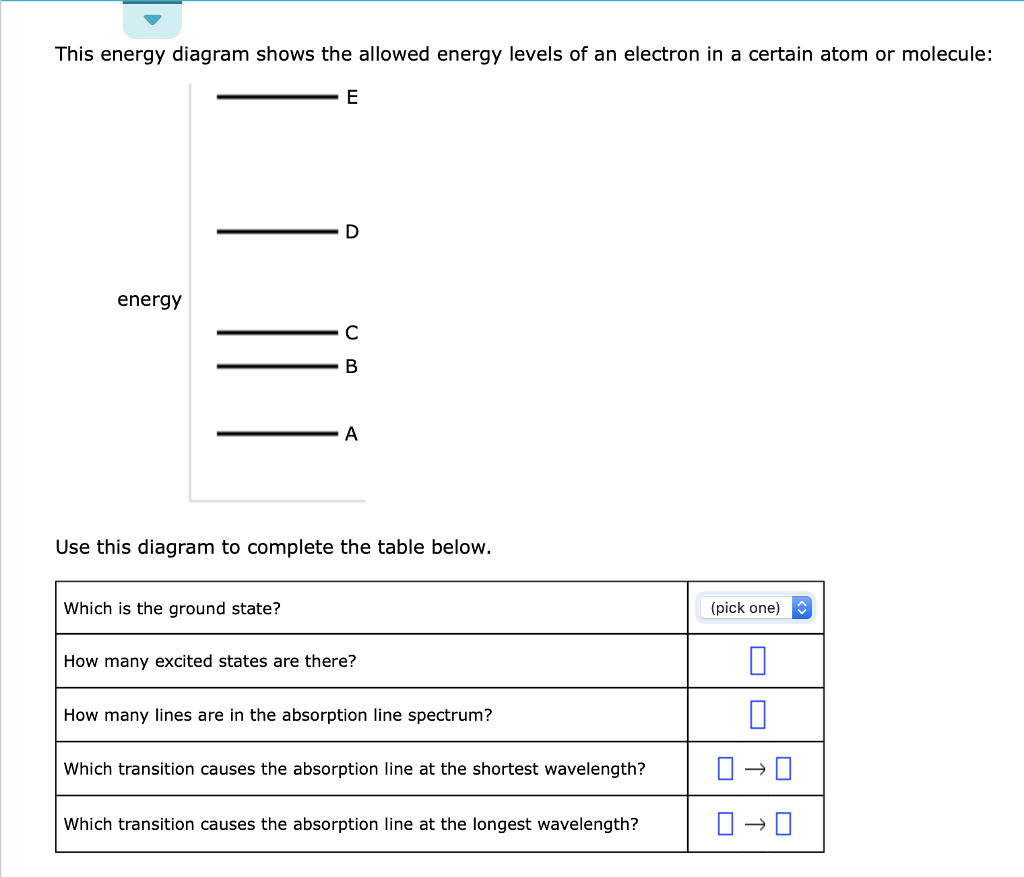
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: Use this diagram to complete the table below. Which transition causes the absorption line at the longest wavelength?
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: energy Use this diagram to complete the table below, Which is the ground state? (pick one) e How many excited states are there? How many lines are in the absorption line spectrum? Which transition causes the absorption line at the shortest wavelength?
Figure 12.7: In the first diagram are shown some of the electron energy levels for the hydrogen atom. The arrows show the electron transitions from higher energy levels to lower energy levels. The energies of the emitted photons are the same as the energy difference between two energy levels.
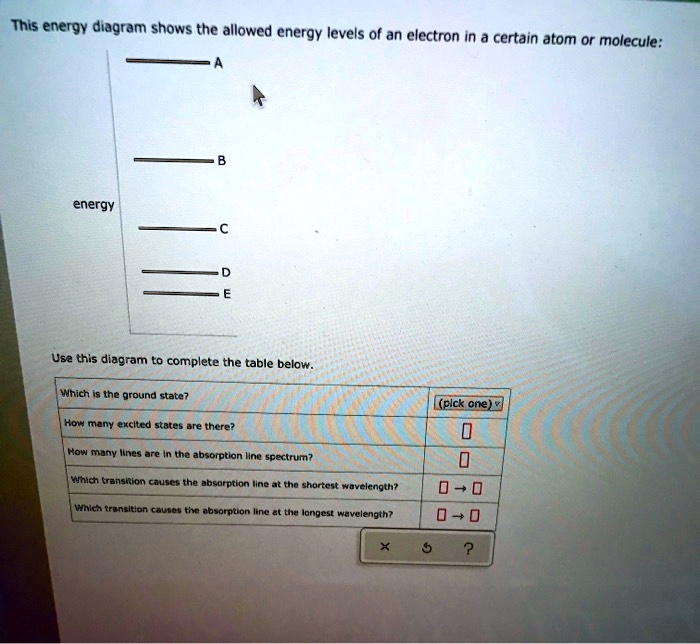
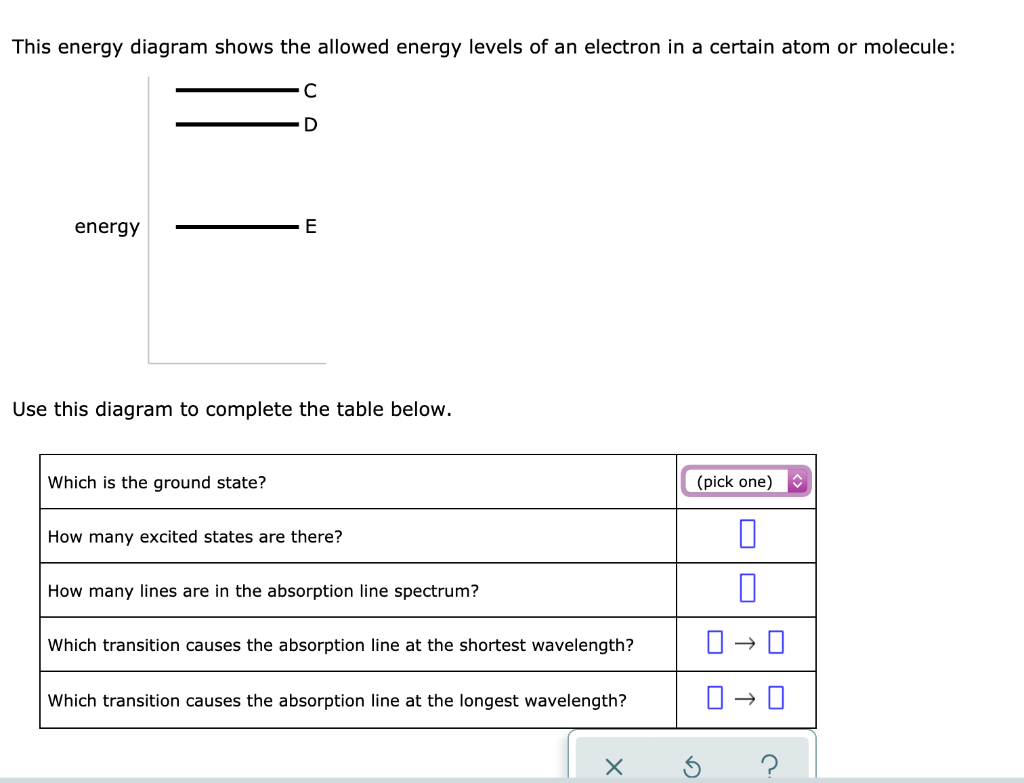
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule:
Quantized energy levels result from the wave behavior of particles, which gives a relationship between a particle's energy and its wavelength.For a confined particle such as an electron in an atom, the wave functions that have well defined energies have the form of a standing wave. States having well-defined energies are called stationary states because they are the states that do not change ...
The x-axis shows the allowed energy levels of electrons in a hydrogen atom, numbered from 1 to 5. The y-axis shows each level's energy in electron volts (eV). One electron volt is the energy that an electron gains when it travels through a potential difference of one volt (1 eV = 1.6 x 10 -19 Joules).
In the hydrogen atom, with Z = 1, the energy of the emitted photon can be found using: E = (13.6 eV) [1/n f 2 - 1/n i 2] Atoms can also absorb photons. If a photon with an energy equal to the energy difference between two levels is incident on an atom, the photon can be absorbed, raising the electron up to the higher level. Sample Problem. The ...
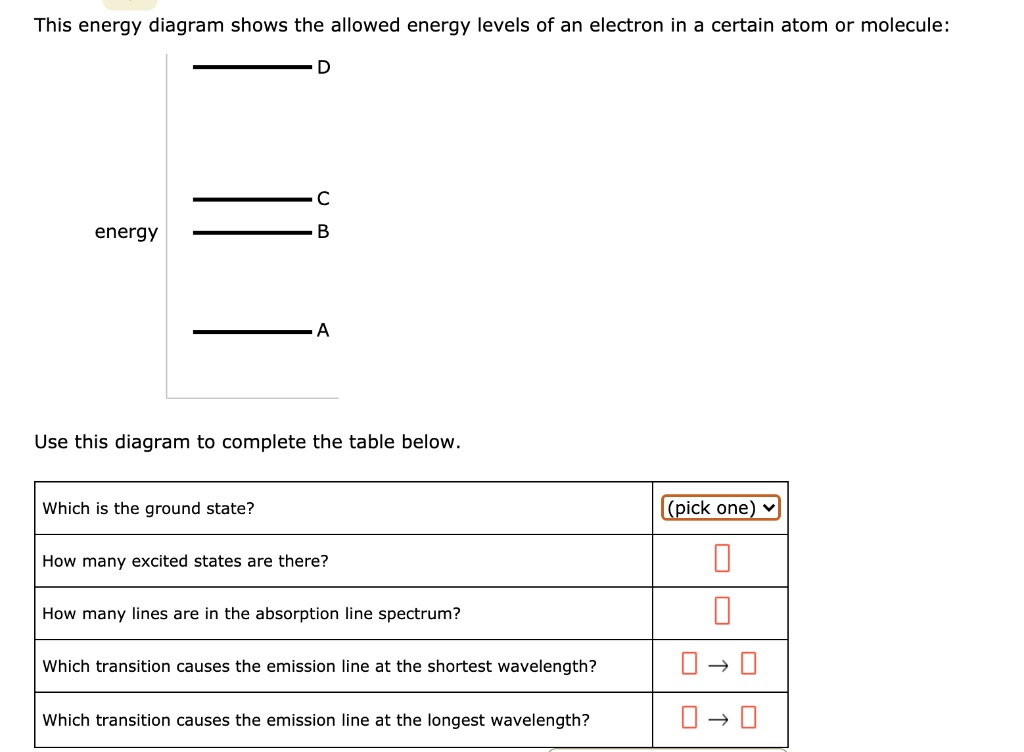
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule:.
Energy Bands in Crystals This chapter will apply quantum mechanics to a one dimensional, periodic lattice of potential wells which serves as an analogy to electrons interacting with the atoms of a crystal. We will show that as the number of wells becomes large, the allowed energy levels for the electron form nearly continuous energy
(From Exam 1) The diagram below shows the allowed energy levels of a certain molecule. A; transition from 푛= 2 to 푛= 1 is accompanied by emission of a photon with wavelength. 휆= 600 nm, as shown in the energy level diagram. When the molecule is in its ground state (푛= 1 ), only certain colors of light can be absorbed.
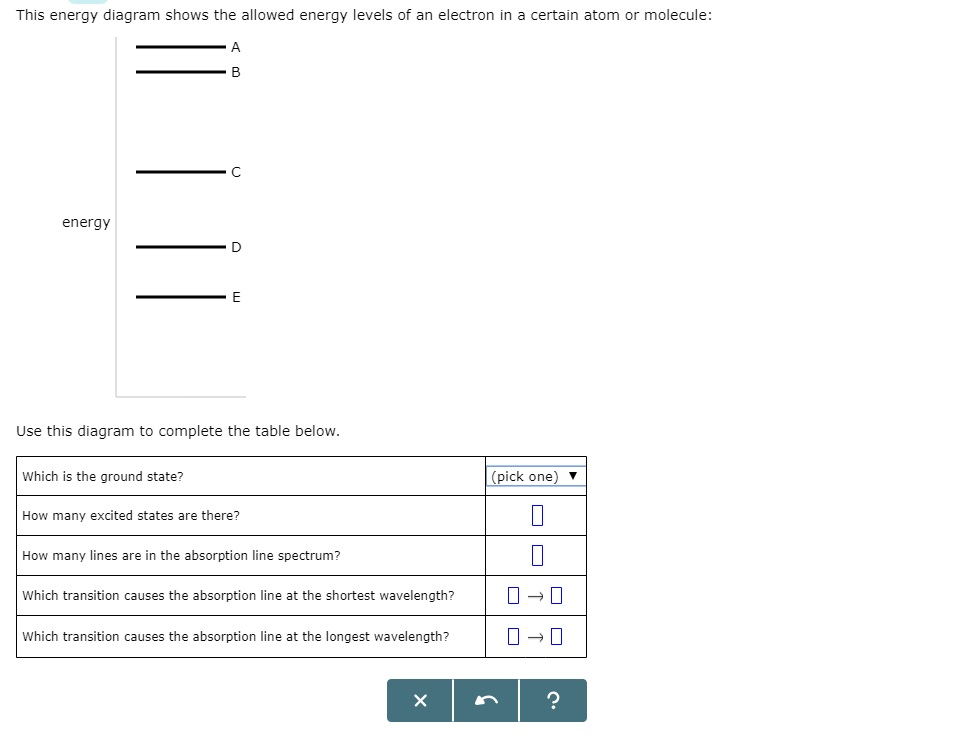
The energy diagram attached shows the allowed energy levels of an electron in a certain atom or molecule. Use the - Answered by a verified Tutor We use cookies to give you the best possible experience on our website.
Depending on the situation, they even neglect the momenta and only look at the allowed energies. This is what we'll do. For example, the figure below shows a two-level atom with a single electron in the lowest energy state. A basic two energy level atom with one electron (yellow) sitting in the lowest energy state.
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: A В C alo energy Ar Use this diagram to complete the table below.
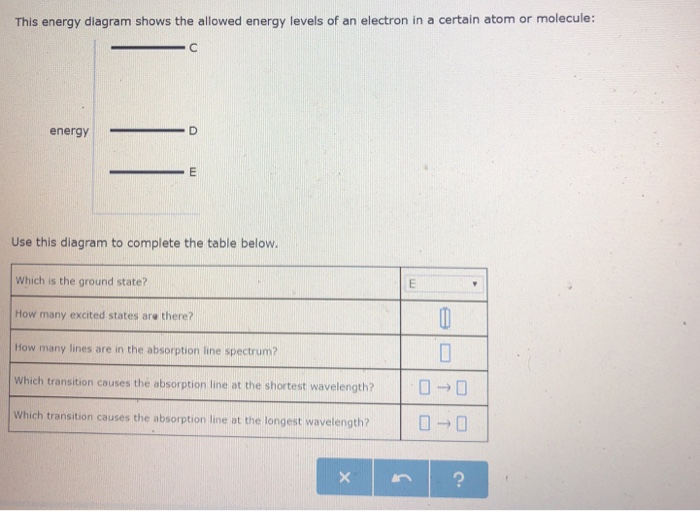
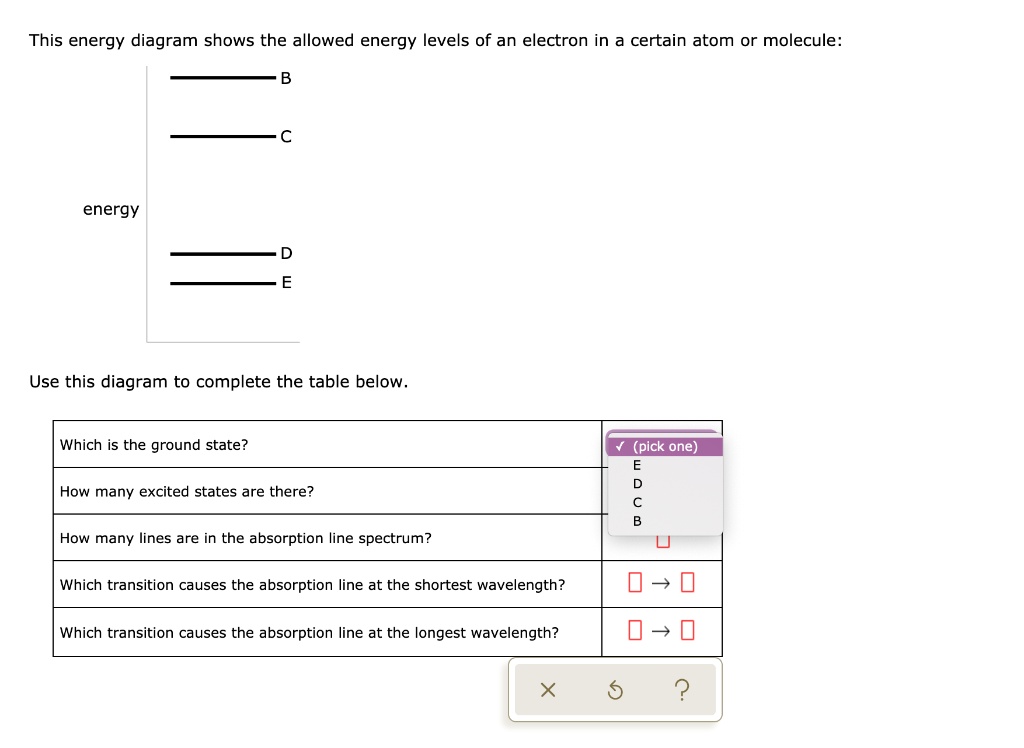
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: с energy B A Use this diagram to complete the table below. Which is the ground state? (pick one) How many excited states are there? How many lines are in the absorption line spectrum? Which transition causes the absorption line at the shortest ...
An electron can't have an energy value half way between two energy levels in an atom. Similarly, a book cannot be placed half way between consecutive shelves. It can only be placed within one shelf.
Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
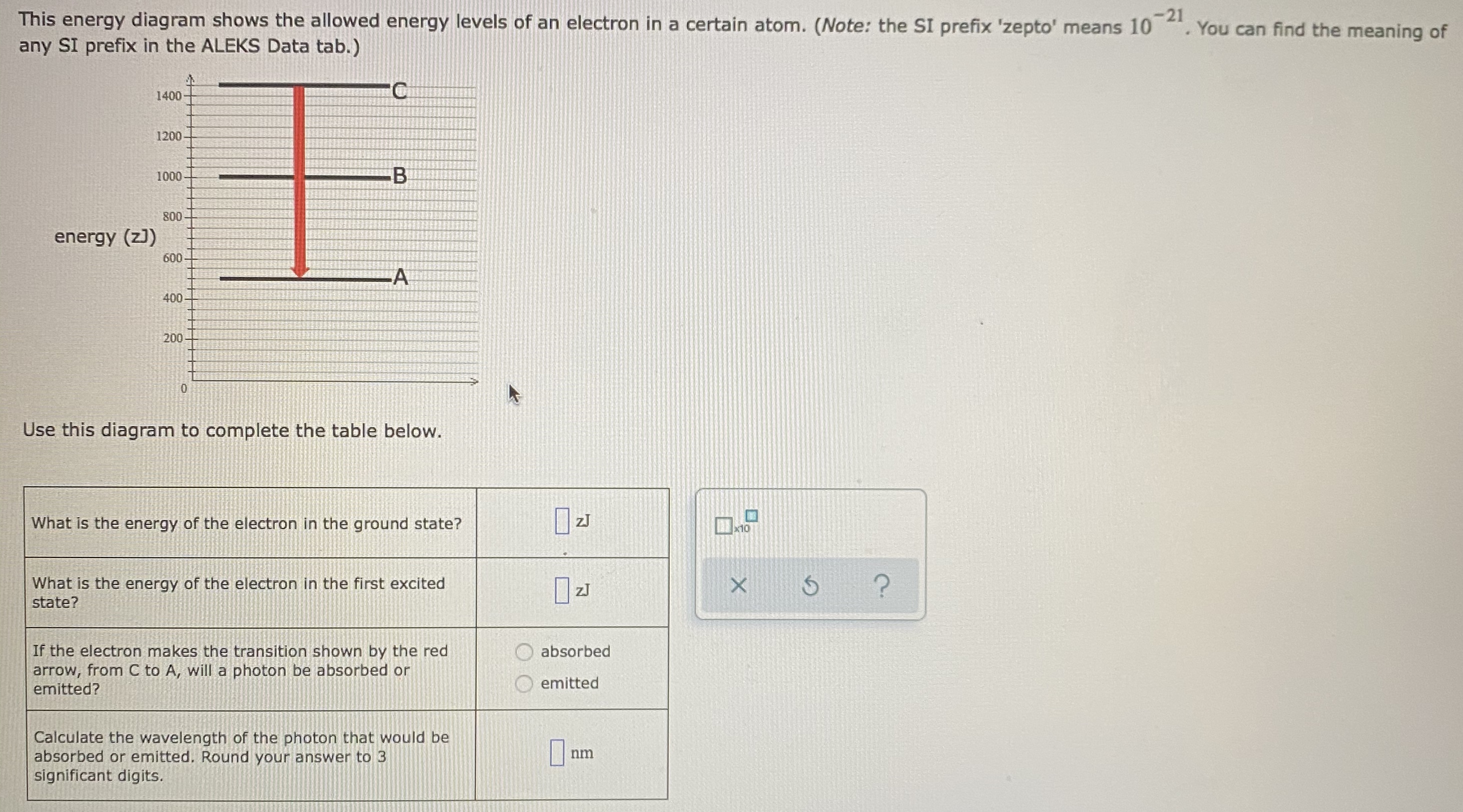
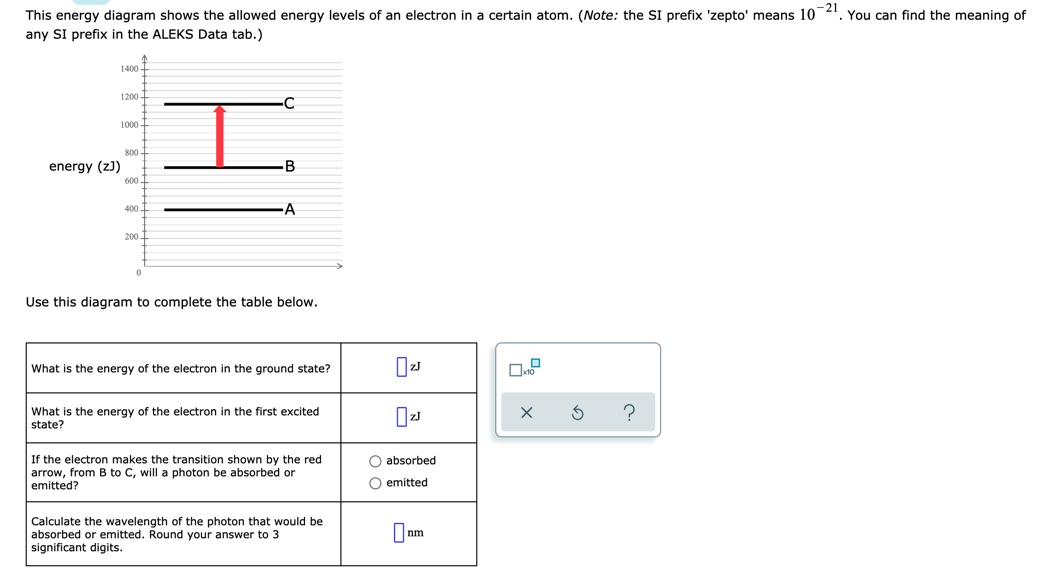
This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10·You can find the meaning of any SI prefix in the ALEKS Data tab.) 1400 1200 alo 1000 800 energy (z)) 600 400 Use this diagram to complete the table below What is the energy of the electron in the ground state? à 10 What is the energy of the electron in the first ...
solved#1894104 - Question: -21 This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: … Show transcribed copy text -21 This earnestness diagram shows the recognized earnestness levels of an electron in a convinced molecule.
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: Use this diagram to complete the table below. Subject: Chemistry Price: 2.85 Bought 3. Share With. This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: Use this diagram to complete the table below.
Sep 07, 2016 · This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule. The lowest energy state for the electrons in an atom or molecule. If it is in the second energy level it must have 34 ev of energy. You can represent electrons as arrows. This energy diagram shows the allowed energy levels of an electron in a certain atom.
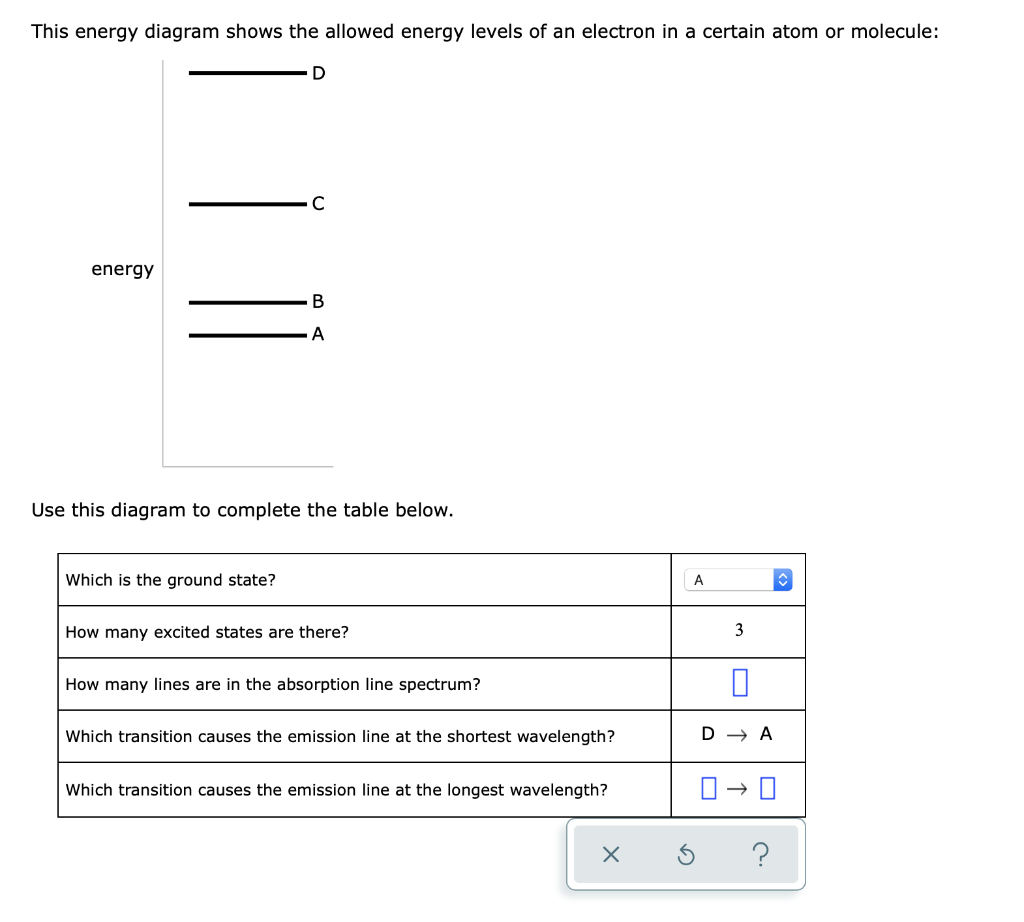
This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: C B energy A Use this diagram to complete the table below. Which is the ground state? ((pick one How many excited states are there? How many lines are in the absorption line spectrum? 0 0 0 0 Which transition causes the emission line at the ...
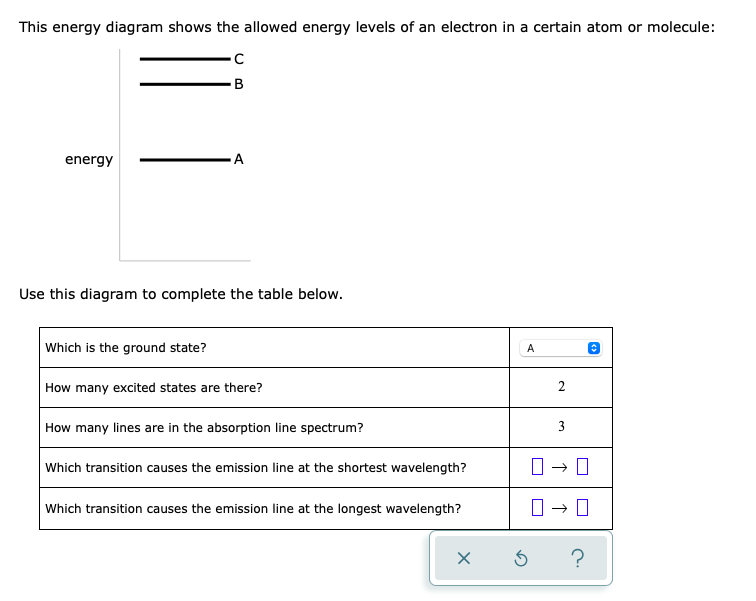
Problem: This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: Use this diagram to complete the table below: What is the ground state?How many excited states are there? How many lines are in the absorption line spectrum? Which transition causes the emission line at the shortest wavelength?
This energy diagram shows the allowed energy levels of an electron in a certain atom. The first electron goes into the 1s orbital filling the lowest energy level first and the second one spin pairs with the first one. You can represent electrons as arrows. If two electrons end up in the same orbital one arrow faces up and the other faces down.
2/28/2021 ALEKS … 1/3 QUESTION This energy diagram shows the allowed energy levels of an electron in a certain atom or molecule: Use this diagram to complete the table below. EXPLANATION You can solve this problem by using some important facts about spectroscopy. First, complete the top two rows of the table by using the fact that the state with the lowest possible energy is called the ...
The diagram shows some of the electron energy levels of an atom. € An incident electron of kinetic energy 4.1 × 10-18 J and speed 3.0 × 106 m s-1 collides with€the atom represented in the diagram and excites an electron in the atom from level B€to€level D. (a)€€€€ For the incident electron, calculate
Question:-21 This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto means 10 any SI prefix in the ALEKS Data tab.) You can find the meaning of energy (z)) Use this diagram to complete the table below.
This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10·You can find the meaning of any SI prefix in the ALEKS Data tab.) 1400 1200 alo 1000 800 energy (z)) 600 400 Use this diagram to complete the table below What is the energy of the electron in the ground state? à 10 What is the energy of the electron in the first ...
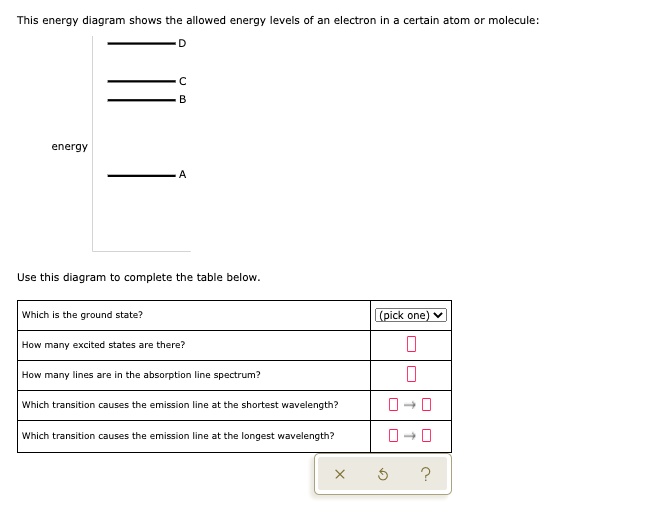
This energy diagram shows the allowed energy levels of an electron In a certain atom or molecule: D -C B energy allo A > Use thi. This energy diagram shows the allowed energy levels of an electron In a certain atom or molecule: D -C B energy allo A > Use this diagram to complete the table below.
The energy diagram attached shows the allowed energy levels of an electron in a certain atom or molecule. Use the - Answered by a verified Tutor We use cookies to give you the best possible experience on our website.
August 2021 - Page 25598 of 32460 - studykind. Question: This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the …. This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix zepto' means 10 . You can find the meaning of any SI prefix in the ALEKS Data ...
🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One...
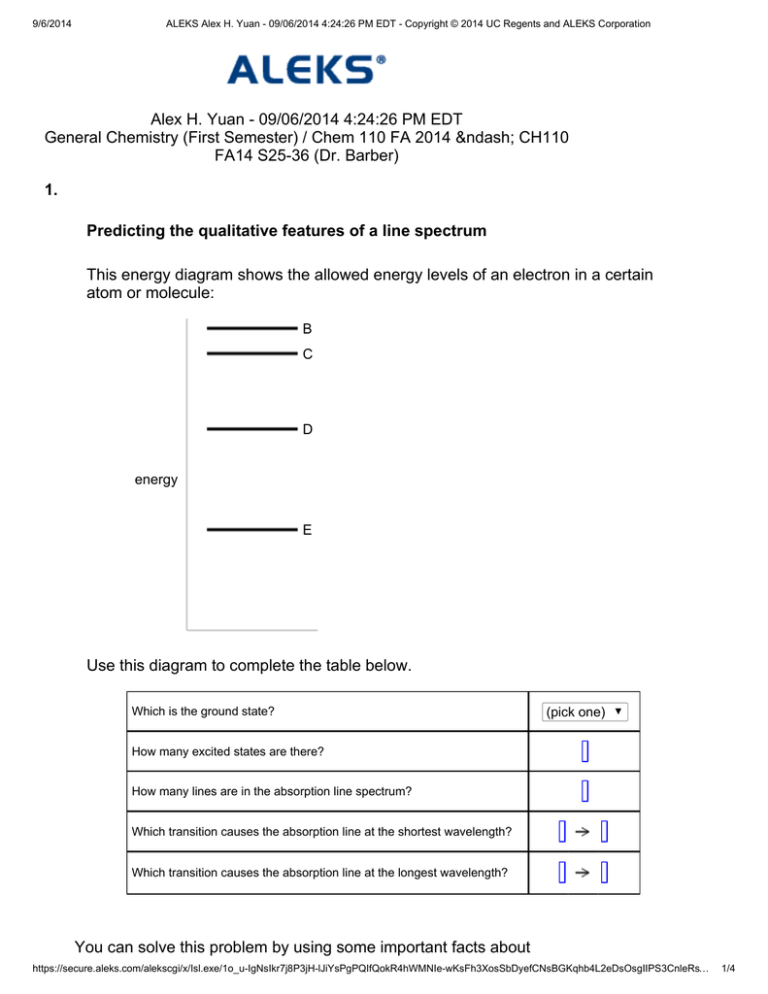
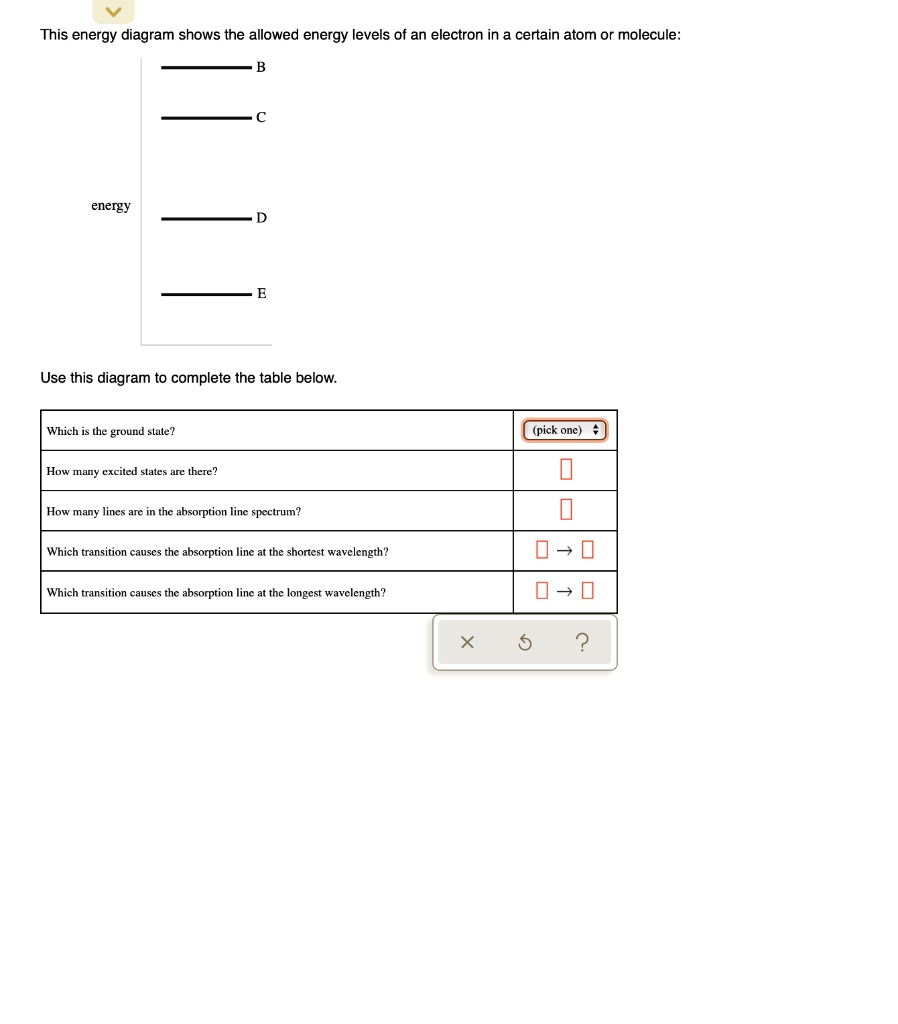








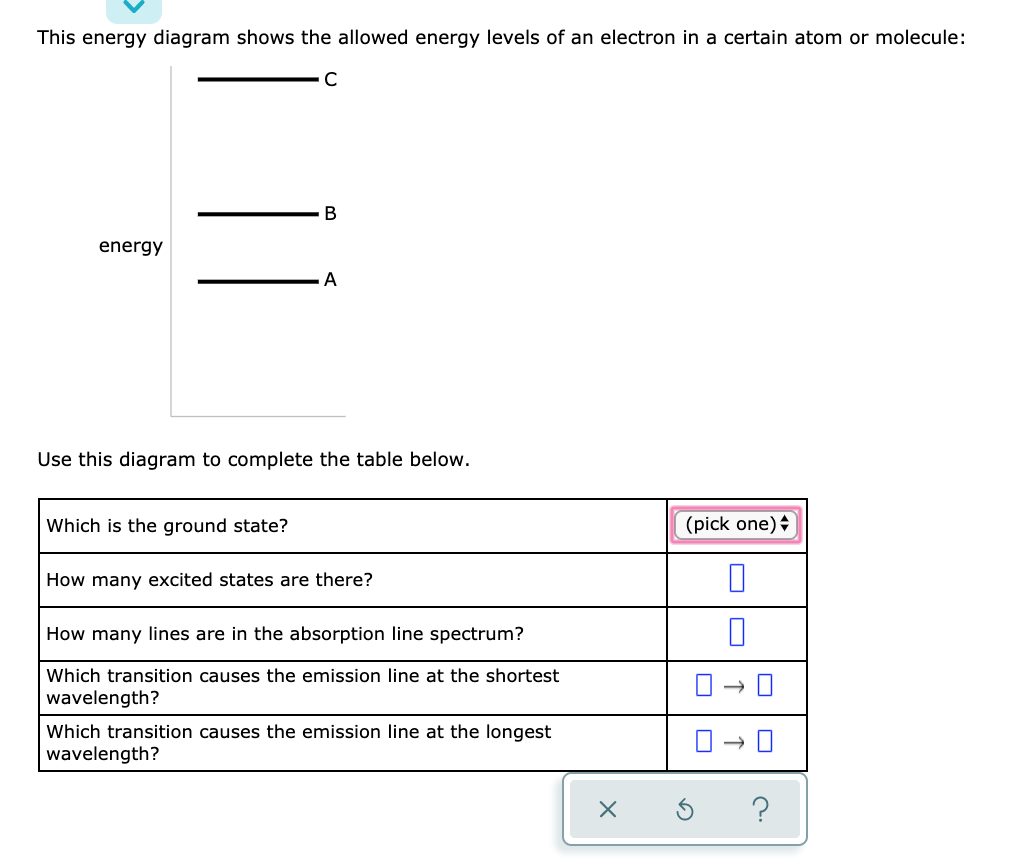









0 Response to "40 this energy diagram shows the allowed energy levels of an electron in a certain atom or molecule:"
Post a Comment