41 lewis dot diagram for cs2
Step-3: Lewis dot Structure for CS2 generated from step-1 and step-2 — The electron dot structure of the CS2 molecule is also known as the CS2 Lewis ...
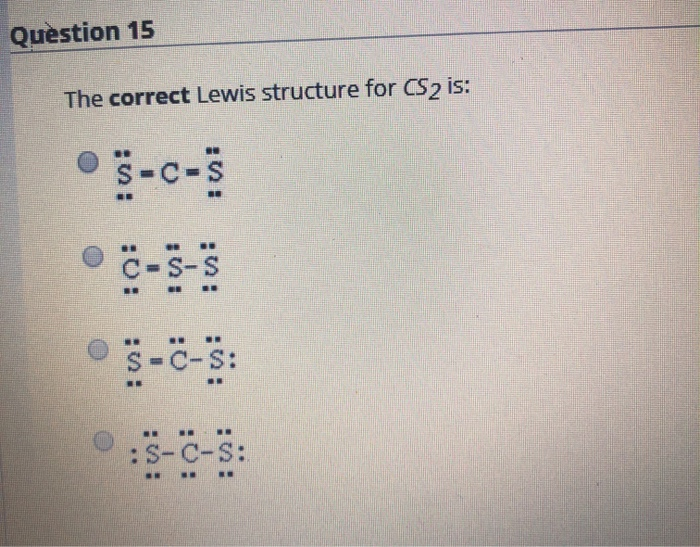
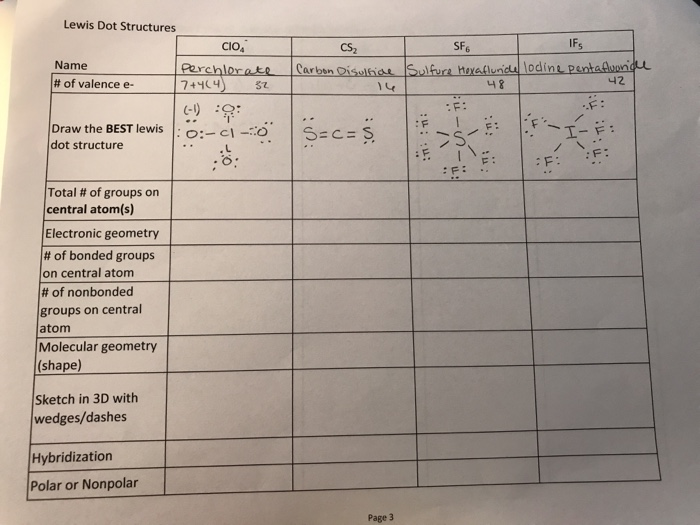
Draw a Lewis Structure for each of the following: 30. NF3 33. ClO4- 31. CS2 32. BH3 34. NH4+ 42. What is the role of the central atom when drawing the Lewis structure for a molecule? All terminal atoms are bonded to it. Homework #9-5 Answers. Read Ch. 9.4 pp. 259-262; Problems pg. 262 #49-53; 59 (omit hybrid).
There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for ...

Lewis dot diagram for cs2
The lewis dot structure for CS2 also predicts many of the properties of the molecule. Due to the presence of large sulfide atoms over for comparison oxygen atoms in CO2 the molecule has a greater capacity for temporary london dispersion forces. As a result there can be more induced dipoles which increases the solubility of CS2.
Draw the Lewis dot structure for. A step-by-step explanation of how to draw the CS2 Lewis Dot Structure Carbon disulfideFor the CS2 structure use the periodic table to find the total numbe. Aluminum 13 3s2 p1 Al 3. I am looking at drawing the lewis structure for the S2- ion. The Lewis structure of S2- is represented by the capital letter S that ...
What is the Lewis dot structure for CS2? In Lewis structure of CS2 molecule, there are 16 valence electrons, out of which four valence electrons are of Carbon, and six valence electrons are from each sulfur molecule. Carbon is the least electronegative molecule and thus comes in the center.
Lewis dot diagram for cs2.
Lewis structures (also known as Lewis dot diagrams, electron dot diagrams,"Lewis Dot formula" Lewis dot structures, and electron dot structures) are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently bonded molecule, as well as coordination compounds.
(a) The sequence of atoms in the CS2 molecule is sulfur to carbon to sulfur. 1 Answer. This site tells all about making lewis dot diagrams. To draw the lewis Dot structure of CS₂(carbon disulfide), we have to find out the valence electrons of carbon and sulfur first.We express valence electrons as dots in lewis dot structure. H:1x2=2 C:4 O:6.
A Lewis dot structure is a drawing of a molecule. The drawing only "works" f0r stable molecules that actually exist. So it's a nice tool to explore how atoms bond into more complex substances. A Lewis dot structure is also called a Lewis structure, a Lewis dot diagram, an electron dot structure, or a dot diagram.
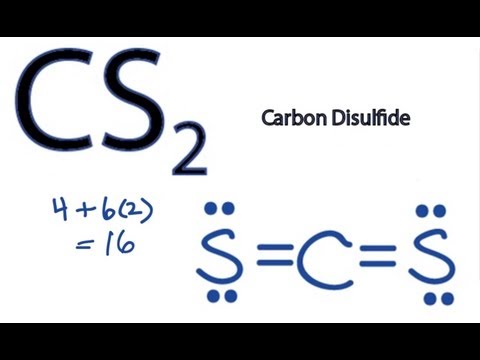
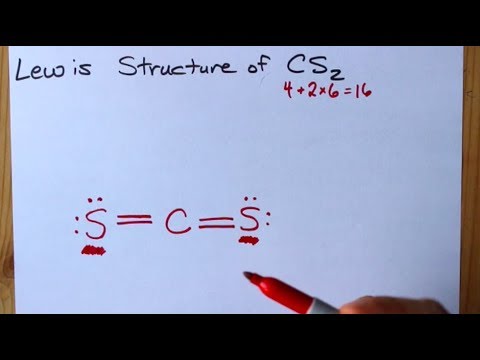
Let's do the Lewis structure for CS2. On the periodic table, Carbon's in group 4, sometimes called 14, so it has 4 valence electrons. Sulfur in group 6 or 16, it has 6. We have two Sulfurs so let's multiply that by 2. Four plus 12: 16 valence electrons. Let's draw it.
Lewis Electron Dot Structures - cs2 lewis structure, simple procedure for writing Lewis structures - Lewis Structures for Carbon Disufide (CS2), lewis structure, lewis dot structure, cs2 lewis structure, carbon disulfide lewis structure, lewis dot diagram, electron dot diagram, Chemistry Net, chemistry tutorial on Lewis structures, online chemistry tutorial, ap, ib, mcat, electron dot ...
What is the Lewis dot structure for CS? There are 16 valence electrons for the CS2 Lewis structure. Carbon is the least electronegative atom and goes in the center of this structure. The Lewis structure for CS2 requires you have double bonds between the Carbon (C) and Sulfur atoms in order to fill the octet of Carbon.
Lewis Dot Structures can be produced by following a sequence of steps. Let's produce a Lewis Dot Structure for: NH 4 + (the ammonium ion). Step 1: Count valence electrons: N = 5 4 x H = 4 x 1 = 4 "+" = -1 Total = 5+4-1= 8 electrons = 4 bonds and lone pairs. Step 2:!Arrange the atoms (identify a central atom, if possible).
The Lewis dot symbol consists of the symbol for the element surrounded by the dot(s). What does the dot or dots represent? ... The Lewis structure for CS2 is... A. Which of these covalent bonds is the most polar? E. Al-I. Which one of the following pure substances will exhibit hydrogen bonding? D. H2O. Sets with similar terms.
Lewis dot structure is a sketchy diagrammatical method of determining how bond formation is occurring within the participating atoms. The structure determines how sharing of the valence electrons is taking place and whether a single, double or triple bond is forming. With this information, molecular geometry, hybridization, and molecular ...
CS2 Lewis Structure. Created by MakeTheBrainHappy. This is the Lewis Dot Structure for Carbon Disulfide (CS2). As we've discussed before you could replace each bond with two electrons which would represent well how through the sharing of electrons each atom achieves a full octet. The combination of four electrons for the bonds and four ...
Lewis dot structure of Nitride ion. Now let us try Lewis dot structure of Sulfide ion ( S 2-).Two negative charges means sulfur atom has gained two electrons so its electronic configuration is with 18 electrons (instead of 16). [Ne]4s 2 4p 6. Valence electrons are 8 (2 in 3s and 6 in 3p) Lewis dot structure of sulfide ion
Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically.
A step-by-step explanation of how to write the Lewis Dot Structure for CS2 (Carbon DiSulfide). For the CS2 Lewis structure, calculate the total number of valence electrons for the CS2 molecule. After determining how many valence electrons there are in CS2, place them around the central atom to complete the octets.
Lewis Structure for CS. 2. (Carbon Disulfide) Commonly Tested Lewis Structures. We draw Lewis Structures to predict: -the shape of a molecule. -the reactivity of a molecule and how it might interact with other molecules. -the physical properties of a molecule such as boiling point, surface tension, etc.
Steps to write the Lewis structure 1. Find the number of valence electrons for each of the atoms in the molecule. The number of valence electrons is usually the same as the group number where the element is located. 3. The number of valence electrons for carbon is 4 (group 4) 4. The number of valence electrons for sulfur is 6 (group 6) 5.
CS2 Lewis Structure|| Lewis Dot Structure for CS2 ||Carbon Disulfide Lewis StructureBlog Post:http://chemistry291.blogspot.com/2019/11/cs2-lewis-structure-le...
CAMEO Chemicals. Pure carbon disulfide is a colorless liquid with a pleasant odor that is like the smell of chloroform. The impure carbon disulfide that is usually used in most industrial processes is a yellowish liquid with an unpleasant odor, like that of rotting radishes. Carbon disulfide evaporates at room temperature, and the vapor is more ...
Problem: Draw the Lewis dot structure for CS2. FREE Expert Solution. We're being asked to draw a Lewis structure for CS 2. To answer this problem, we must: Step 1. Find the center atom of this compound. Step 2. Determine the total valence electrons present. Step 3. Draw the Lewis structure for the compound.
Lewis dot structure. The Lewis dot structure for Strontium Sulfide is simply Sr-S. The S atom has 3 nonbonding electron pairs around it. Every element in the second group (column) has 2 dots in the electron dot structure (also known as the lewis dot structure): Beryllium, Magnesium, Calcium, Strontium, Barium, and Radium.
The lewis dot structure for CS2 also predicts many of the properties of the molecule. C Draw the 3D structure and use polarity arrows to show whether the compound is polar or nonpolar. B The rightmost bond between C and S is a triple bond. Draw the Lewis dot structure for CS2.
A step-by-step explanation of how to draw the CS2 Lewis Dot Structure (Carbon disulfide).For the CS2 structure use the periodic table to find the total numbe...
CS2 Lewis Structure. Answer: CS2 Lewis structure (carbon disulfide electron dot structure) is that type of diagram where we show the total 16 valence electrons of CS2 as dots , or dots and dashes (-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash (-) or dots ( ) but a lone pair of two electrons is ...
CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape. CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure. ぷっくりお花のキーホルダー ...
The Lewis Structure for Li is Li with one dot to the right of the element. People also asked What is the number of shared electrons in the Lewis dot structure for CS2?



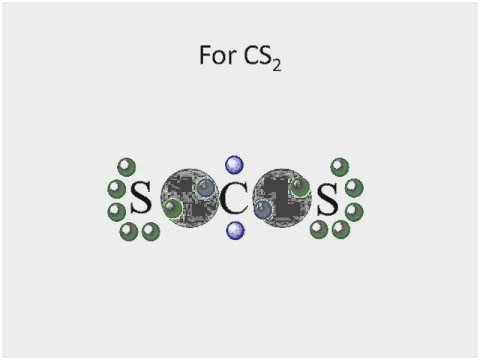






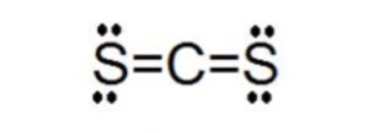
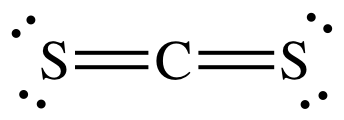
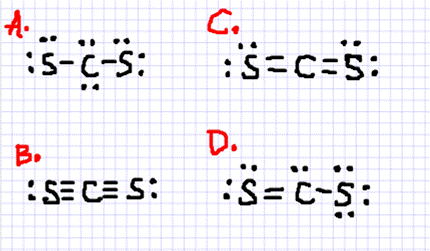








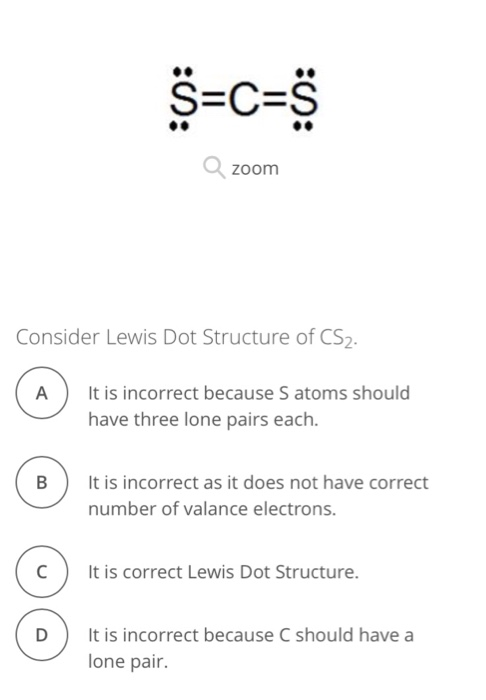
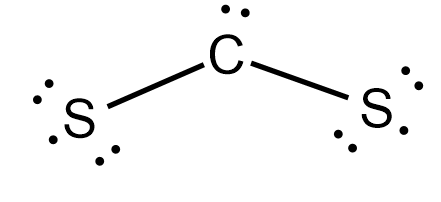




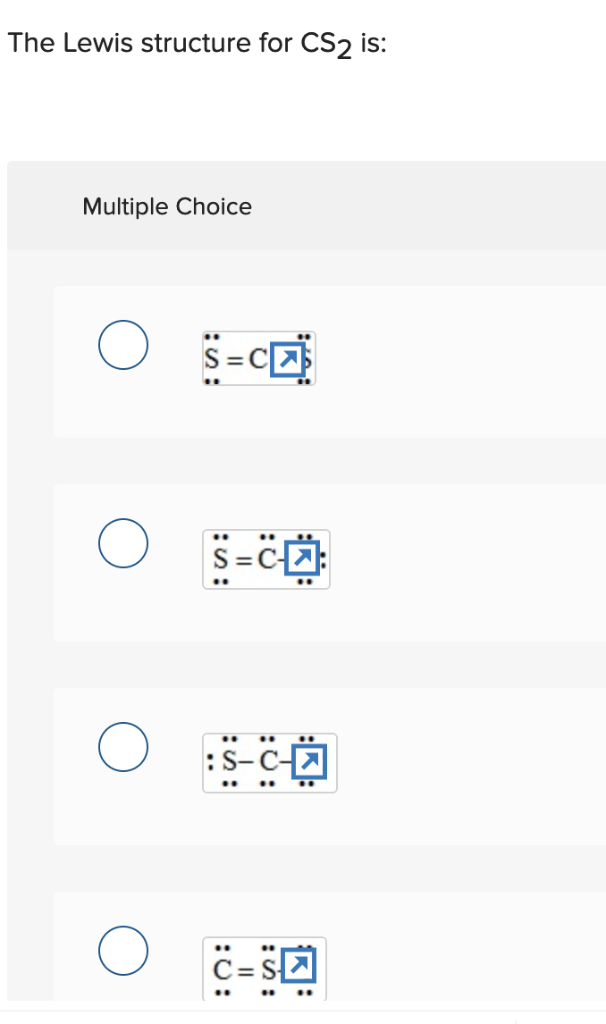


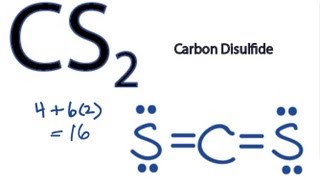
0 Response to "41 lewis dot diagram for cs2"
Post a Comment