44 atomic orbital diagram for nitrogen
nitrogen bohr diagram Nitrogen Dioxide (NO2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. bohr diagram for nitrogen. The second and third orbital can hold a maximum of 8 electrons. 18 p. 22 n. Bohr diagram of nitrogen.
In nitrogen MO , there is a slight change in MO diagram when compared to the MO diagram s of O 2, F 2 molecules.Here,t he σ 2p orbital is higher in energy than the π 2p orbital. This is because, the σ 2p orbital in oxygen / fluorine is more ... Atomic Orbital Diagram For Chlorine Wiring Site Resource
The electron configuration of potassium and its atomic number. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. A truncated version of the Periodic Table, showing Lewis Find the number of electrons, protons and neutrons. How to draw a bohr model for calcium. Write the total number […]
Atomic orbital diagram for nitrogen
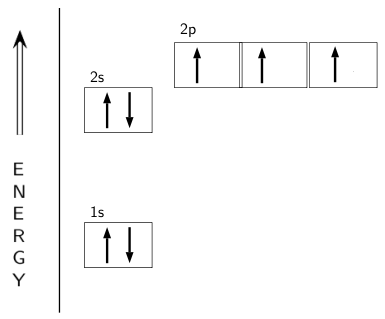
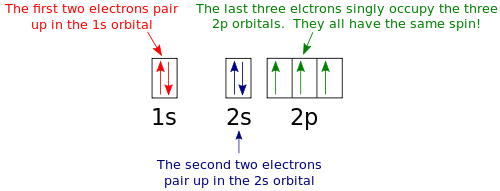
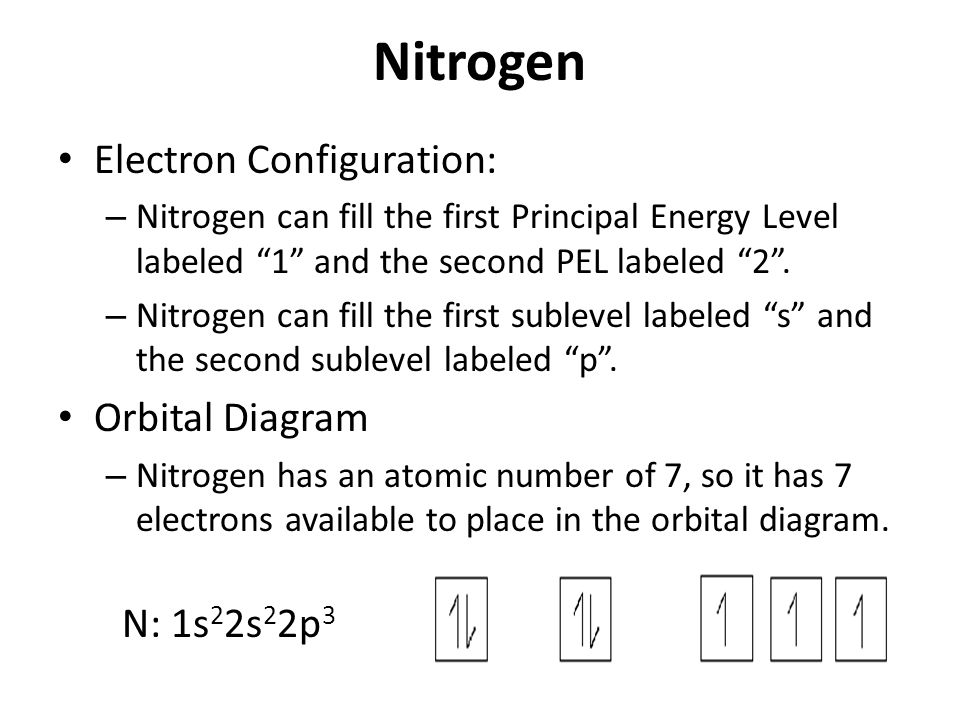
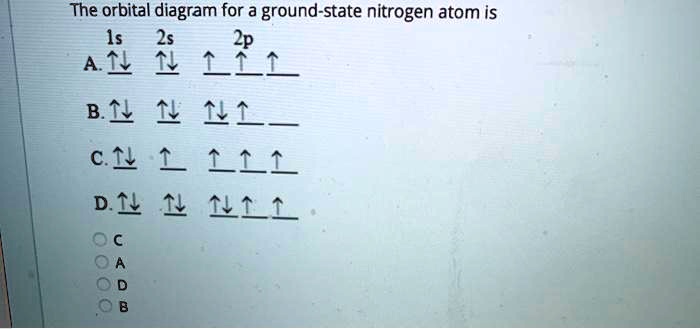
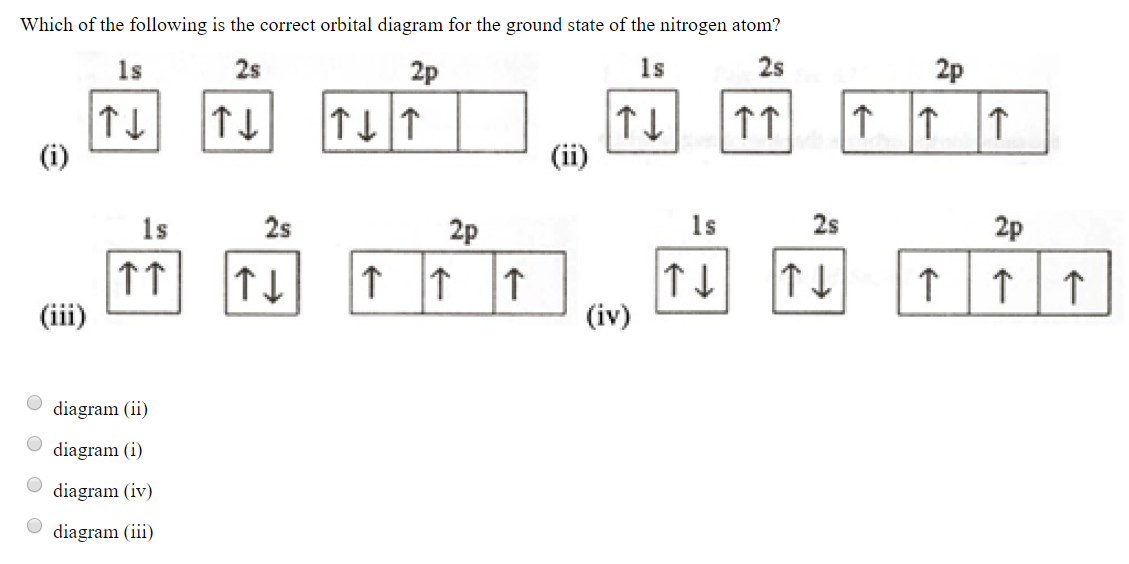
Many times it is necessary to see all the quantum numbers in an electron configuration this the purpose of the orbital diagram. Nitrogen atomic number 7 fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals in accordance with Hunds rule.
Bohr Rutherford Diagram For Nitrogen. Bohr diagram s show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are. Bohr atomic model of a nitrogen atom. Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed by the Danish. Bohr Ruthford ...
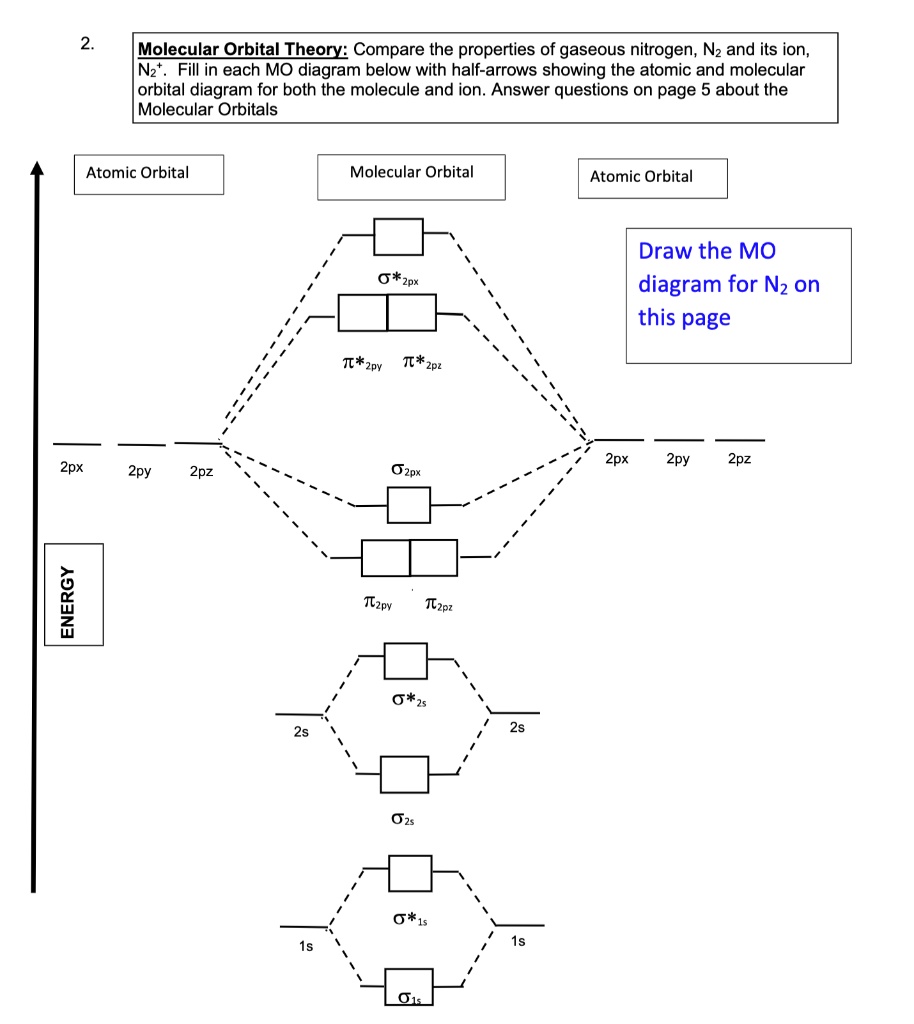
Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. Whilst this is the MO diagram for N₂: If we compare such diagram s for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂...
Atomic orbital diagram for nitrogen.
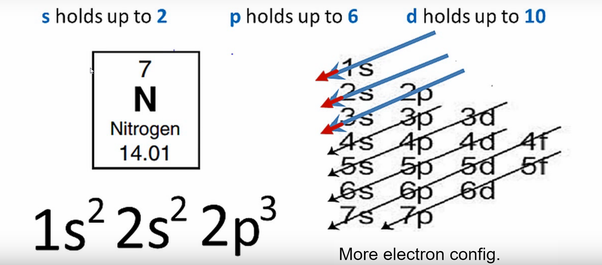
In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons ...Oct 24, 2016 · Uploaded by Wayne Breslyn
4 Bellwork The electron configuration of nitrogen is 1s2 2s2 2p3. What is the atomic number of nitrogen? How many electrons are in a nitrogen atom? Write the orbital notation for nitrogen. 5 Bellwork - 1/27 The electron configuration of boron is 1s2 2s2 2p1. What is the atomic number for boron?
Oxygen-15 is synthesized through deuteron bombardment of nitrogen-14 using a cyclotron. Elements with the same electrons or electron configurations are said to be isoelectronic. Draw The Atomic Structure Of Oxygen Ion O 2 Brainly In . Oxygen Electron Configuration O With Orbital Diagram
The two electrons of 1s2 in the Nitrogen atom take part in σ2s MO. The oxygen atoms contribute to 2 lone pairs each. The remaining electrons in p orbitals of N and O form the σ2px, 𝜋2py, 𝜋2pz, and σ*2s. Conclusion NO2 is one of the most common heteronuclear diatomic molecules.
According to Molecular Orbital (MO) Theory, two atoms mix their orbital s to form one that is spread out over both Figure1: MO diagram for H2. After a preliminary check with He2 and He2+, self‐consistent field calculations have been carried out for the nitrogen and carbon monoxide.
Write the orbital diagram of carbon before sp3 hybridization. Write the orbital diagram of carbon before sp3 hybridization. The carbon atoms form a σ sp 2 sp 2 bond with each other by using sp 2 hybrid orbitals. The resulting hybrid orbitals are called sp hybrids. Use the buttons at the top of the tool to add orbitals.
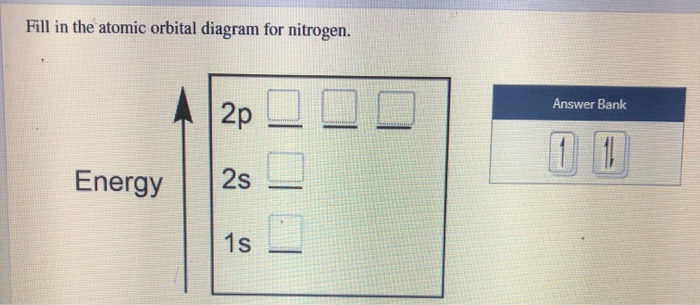
Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so ...
Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist Jean ...
The molecular orbital diagram of the BeCl2 molecule is draw n by the combinat ion of Beryllium atomic orbital s and chlorine group orbital s. As the re are two chlorine atoms and hence, first the y combine to for m group orbital s. The electronic configurat ion of Cl is [Ne] 3s23p5. Now, the 3s atomic orbital of one chlorine atom will combine ...
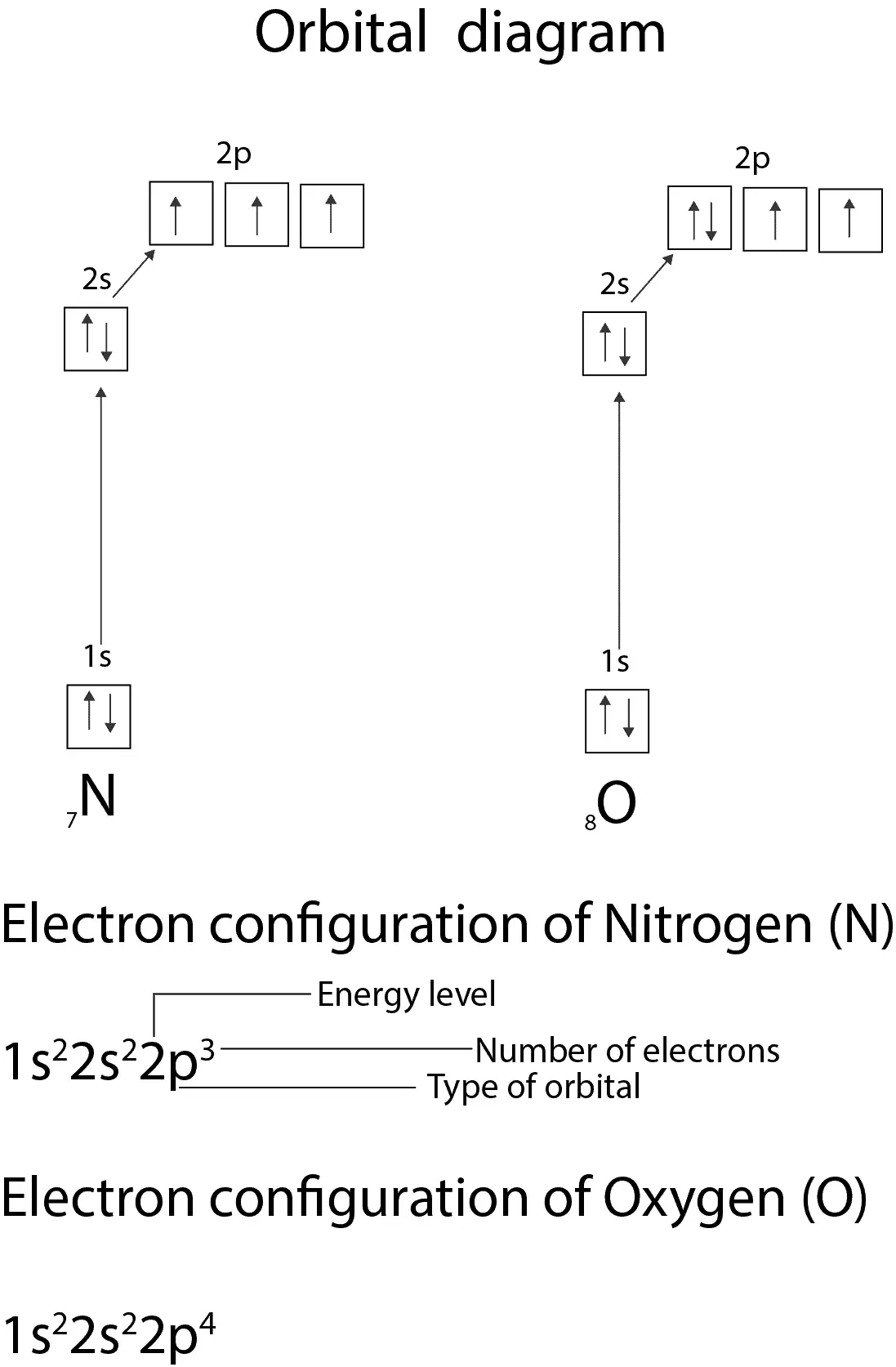
Write the electron configuration of NO molecule in the ground electronic state based on this energy diagram. 6o" Sx 20" 21 Y atom orbitals lo NO molecular ...1 answer · 0 votes: Nitrogen rightarrow 7 rightarrow 1 s^2 2 s^2 2p^3 orbital diagram (2) Ni rightarrow 28 rightarrow 1 s^2 2s^2 2p^6 3s^2 3p^6 3d^8 4s^2 orbital diagram:
There are alkali, argon, electron, nitrogen, phosphorus, silicon, and sulfur orbital diagram s that you can save for free. Orbital diagram s are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagram s. According to the Auf Bau Principle, each electron occupies the lowest energy orbital.
Nitrogen has exactly half filled p-subshell i.e 1s 2 2s 2 2p 3 while ... of inert pair effectwith suitable examples. =The inert pair effect is the tendency of the electrons in the outermost atomic s orbital to remain unionized or unshared in compounds of post-transition metals.The term inert pair effect is often used in relation to the ...
Atomic number of nitrogen = 7. Therefore number of electrons = 7. Thus, electronic Above orbital diagram shows the electron configuration of nitrogen Click here to get an answer to your question ️ Above orbital diagram shows the electron configuration of nitrogen atom. Which rule does not support this?
Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from solving the Schrodinger's equation for Bohr's hydrogen atom. Hence, many of the rules that we use to describe the electron's ...
A: the central atom, here Nitrogen will be A. X stands for the surrounding atoms for nitrogen, ∴ n = 3. E stands for lone pairs of the central atom, ∴ n = 1. The resultant notation will be AX3N1. Let us look at the VSEPR notation chart. According to the VSEPR chart, the molecular geometry of nitrogen trifluoride is trigonal bipyramidal.
Jan 21, 2021 — When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next ...
There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Apart from that one ...Feb 15, 2021 · Uploaded by Wayne Breslyn
The first two electrons of nitrogen enter the 1s orbital and the next two electrons enter the 2s orbital. The s-orbital can have a maximum of two electrons. So, ...Jul 5, 2021 · Uploaded by Wayne Breslyn
35 the diagram shows two product supply curves. it... 37 kubota zd331 parts diagram; 40 label the indicated parts in this diagram of a ... 41 refer to the diagram. by producing at output le... 41 surge protector wiring diagram; 38 nitrogen atomic orbital diagram; 35 ge electric motor parts diagram; 40 2005 dodge ram 1500 4.7 belt diagram
Note that in the orbital diagram, the two opposing spins of the electron can be visualized. This is why it is sometimes useful to think about electron configuration in terms of the diagram. However, because it is the most time consuming method, it is more common to write or see electron configurations in spdf notation and noble gas notation.
Orbital Energies and Atomic Structure. The energy of atomic orbitals increases as the principal quantum number, \(n\), increases. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of \(l\) differ so that the energy of the orbitals increases within a shell in the order s < p < d < f.
If we look at the correct electron configuration of the Nitrogen (Z = 7) atom, a very important element in the biology of plants: 1s 2 2s 2 2p 3 We can clearly see that p orbitals are half-filled as there are three electrons and three p orbitals.
It is related with the energy level of a given electron configuration. An ultrafast approach of determining the atomic term symbols of the s2 p2 d2 f2 electron configurations is introduced. 2S 1 is the spin multiplicity. 2S1LJ where 2S 1 is the spin multiplicity and S is the total spin angular momentum is the total orbital angular momentum.
The p orbital can hold up to six electrons. Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s orbital. The atomic number of sodium is 11. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital. Therefore the Calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p ...
























0 Response to "44 atomic orbital diagram for nitrogen"
Post a Comment