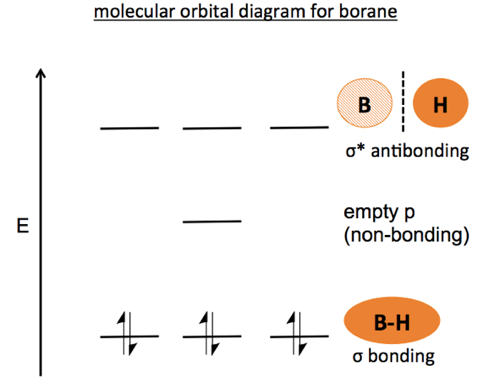
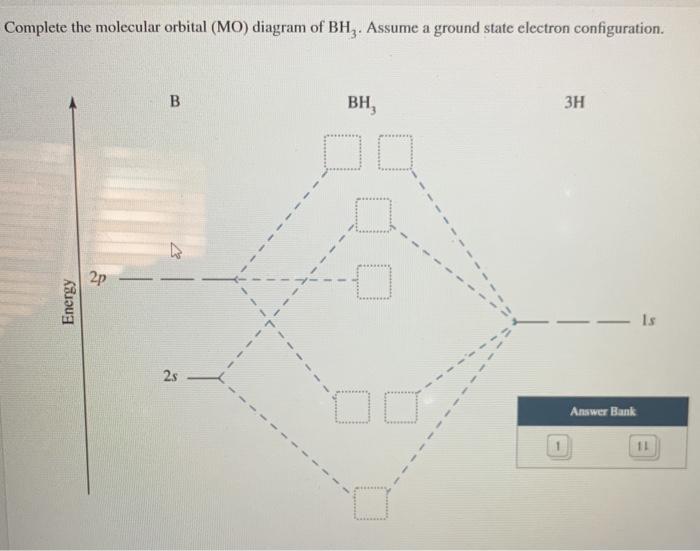
44 bh3 molecular orbital diagram
That is, when atomic orbitals interact to form molecular orbitals, the AO interaction is strong ---- when there is good spatial overlap. Overlap is a central concept in molecular orbital theory. The idea is that if you have atoms that are far apart from each other in space, then their wave functions have dropped off exponentially and are not ...
Answer (1 of 2): Because there is a possibility of electron pair donated from any other compound to lower its (remember the octen rule?) and subsequently the total energy. The pair donating molecule could be water in air moisture or another BH3 molecule. In the latter case three-center bonds HBH ...
In molecular BH3 the molecule is planar with bond angles of 120o so the hybridisation of the central boron atom is sp2. In the dimer B2H6 the molecule has two bridging hydrogens. The hybridisation ...

Bh3 molecular orbital diagram
Polyatomic Molecular Orbital Theory Transformational properties of atomic orbitals Atomic orbital Transforms as s x2+y 2+z 2 px x py y pz z dz2 z2, 2z 2-x2-y2 dx2-y2 x2-y2 dxy xy dxz xz dyz yz S py • When bonds are formed, atomic orbitals combine according to their symmetry. • Symmetry properties and degeneracy of orbitals and bonds can be ...
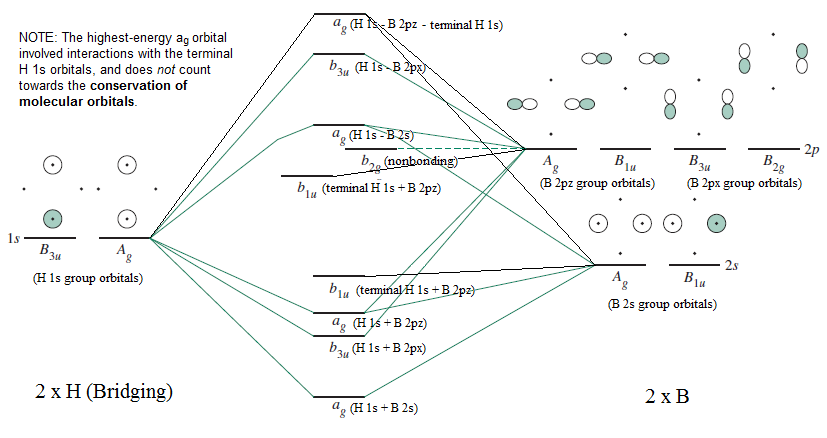
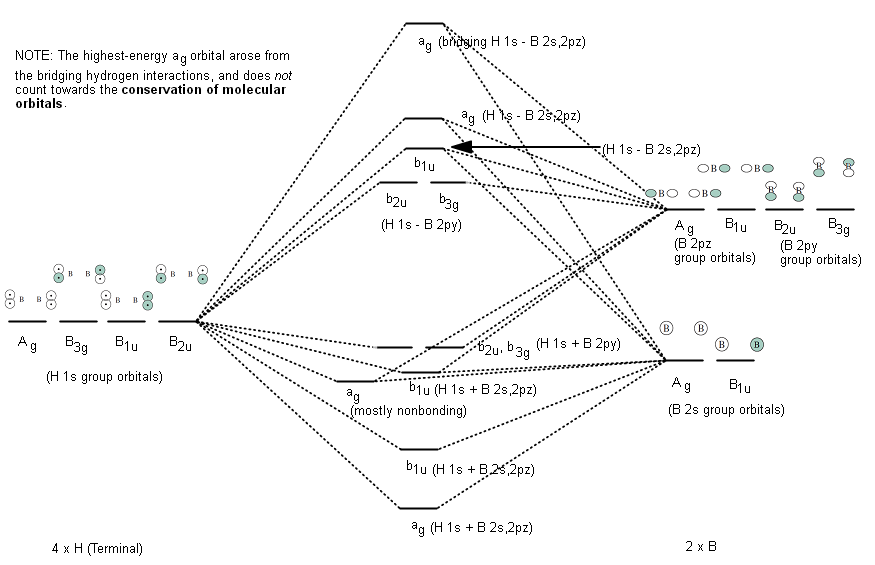
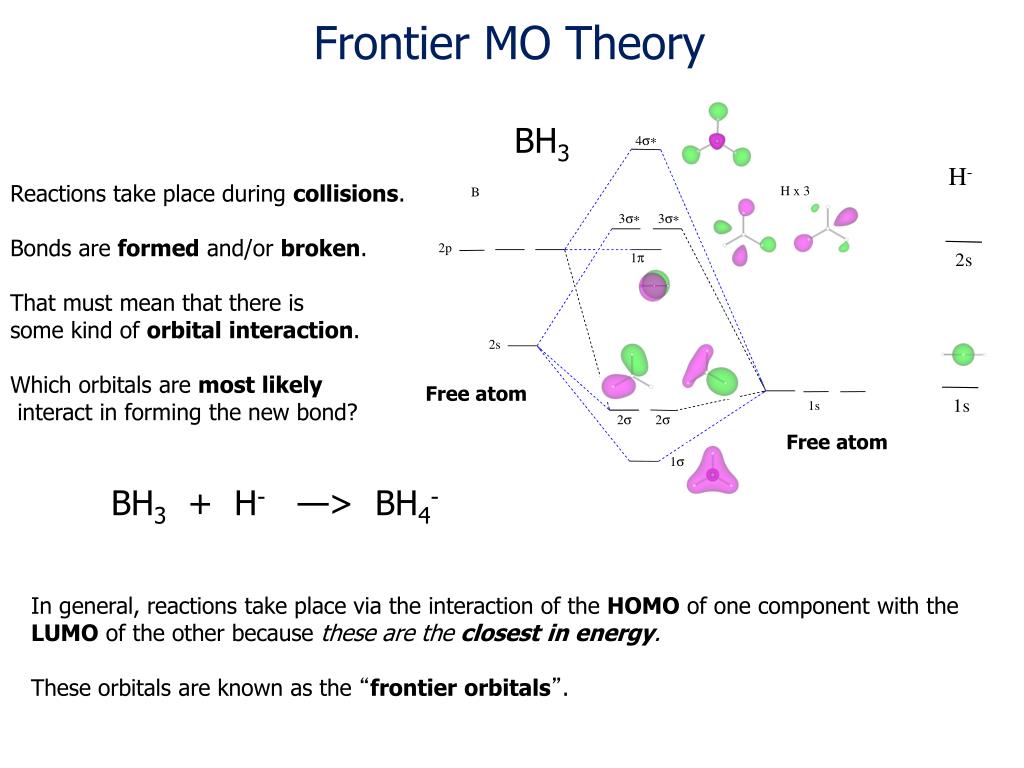
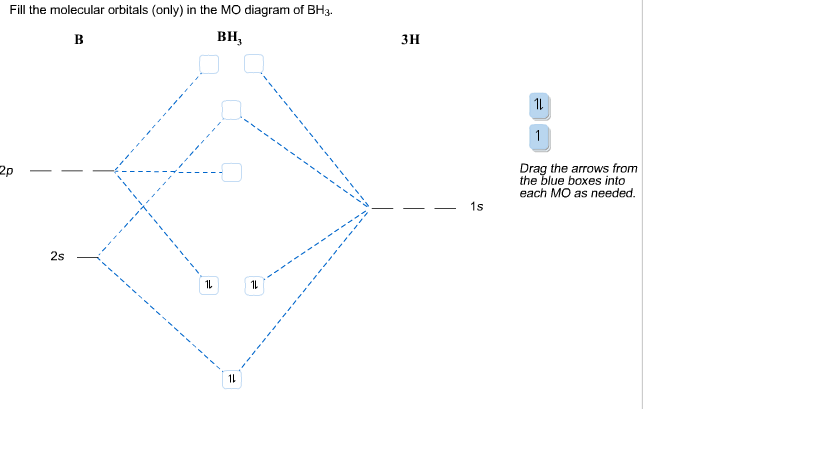
The following figures show how the computer parses the total electron density into SCF molecular orbitals (presented in order of increasing energy, omitting the lowest MO which is mostly the 1s core orbital on B). Since there are 7 valence-level atomic orbitals (1s on each of three H atoms plus 2s and three 2p AOs on B) there are 7 orthogonal low-energy molecular orbitals that can be made from ...
An advanced molecular orbital diagram of BH3 (borane) for the inorganic or physical chemistry student.
Bh3 molecular orbital diagram.
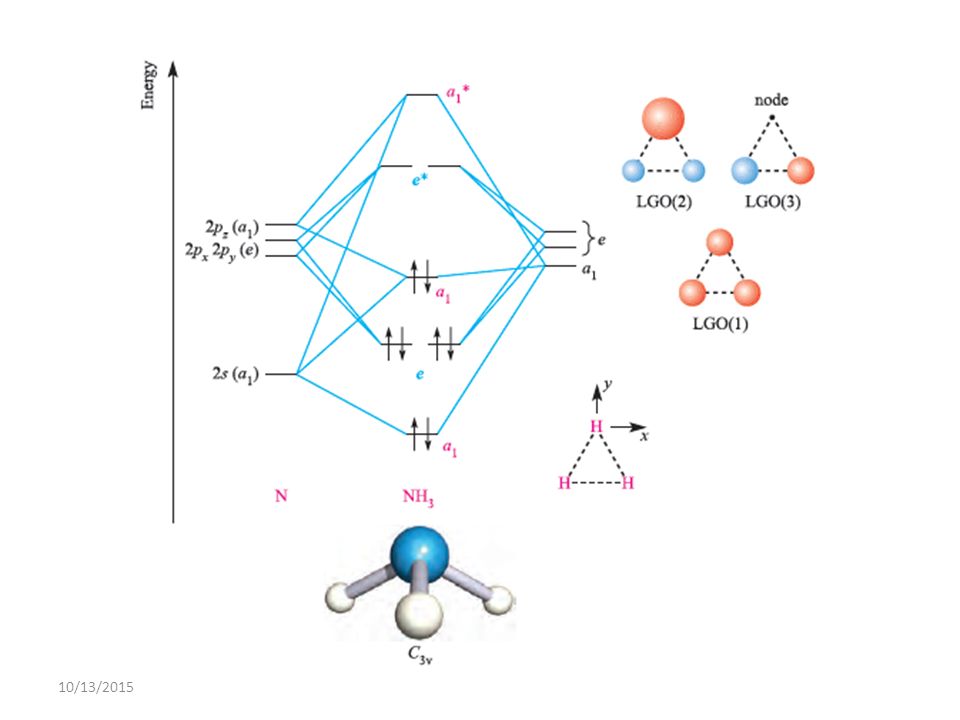
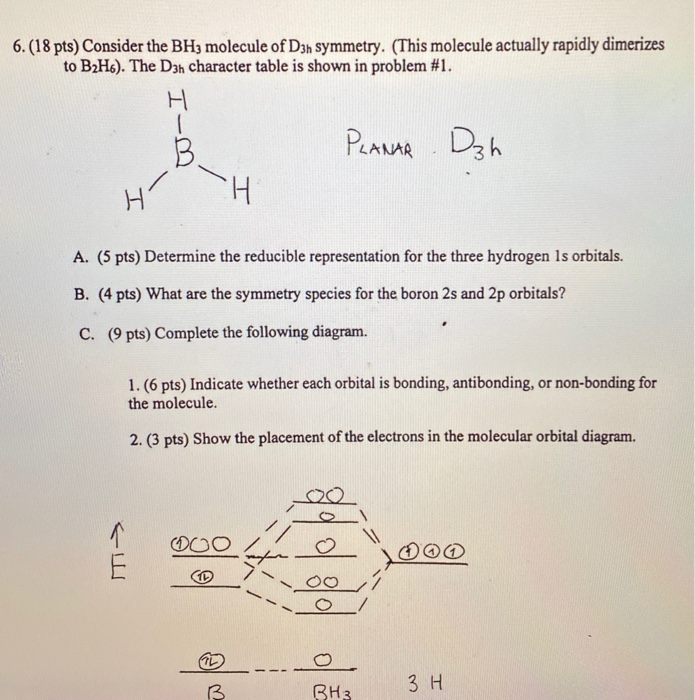
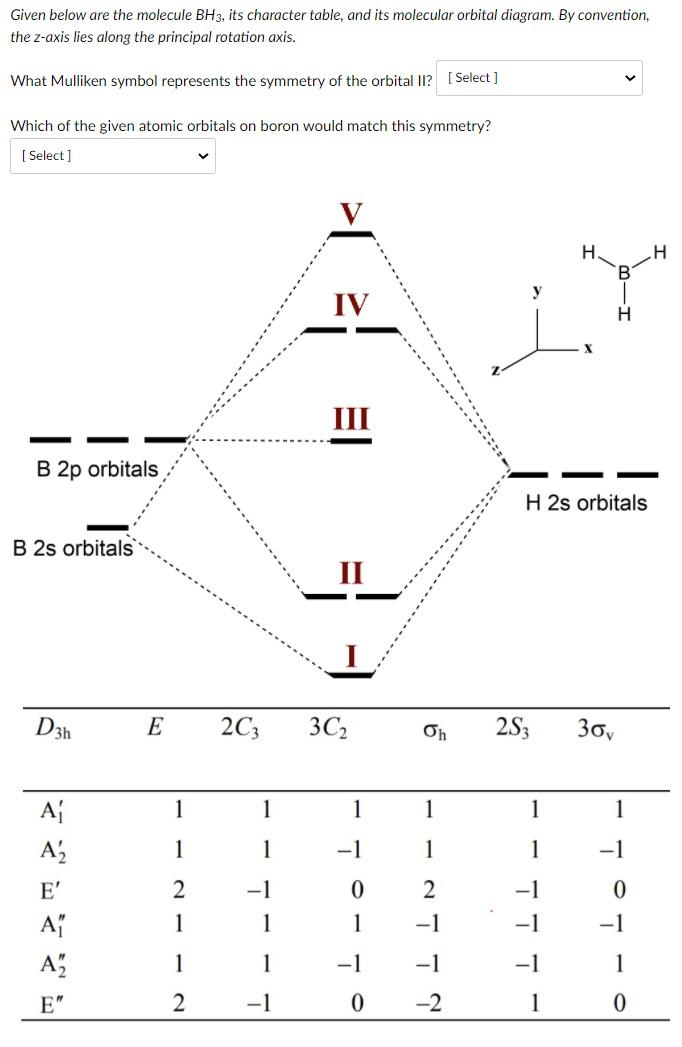
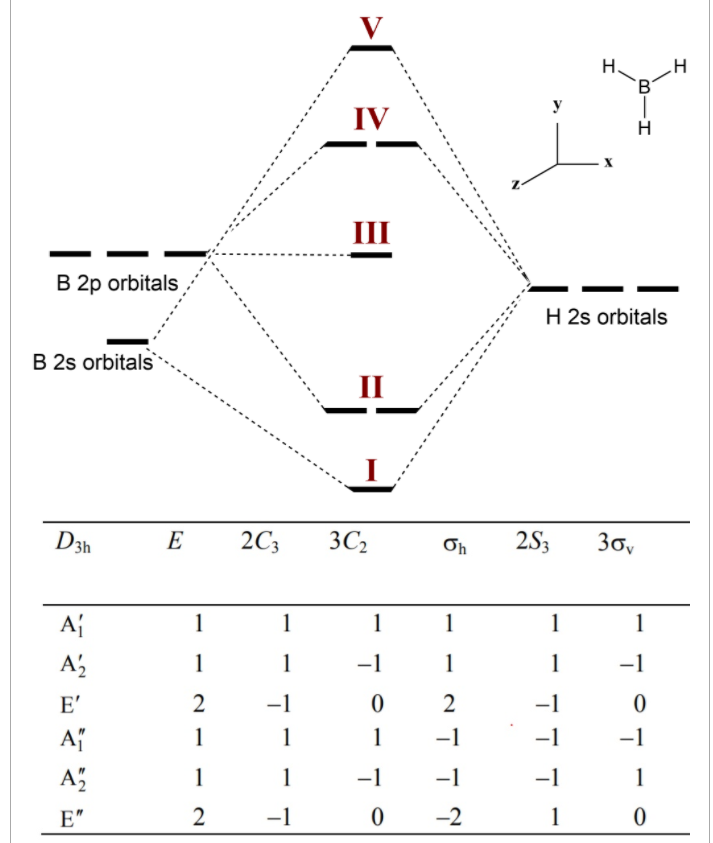
FIGURE2: Character table for the the point group D3h. B atom in BH3: +s-orbital: with the shape of the sphere, its function is x 2 +y 2 +z 2.Therefore, 2s orbital has a 1 ' symmetry +p-orbital: has 3 orbitals , p x, p y, p z.Therefore, 2p z orbital has a 2" symmetry. 2p x and 2p y orbital are degenerate and have e' symmetry. 3 Hydrogen atoms in BH3: (Ligand group orbitals)
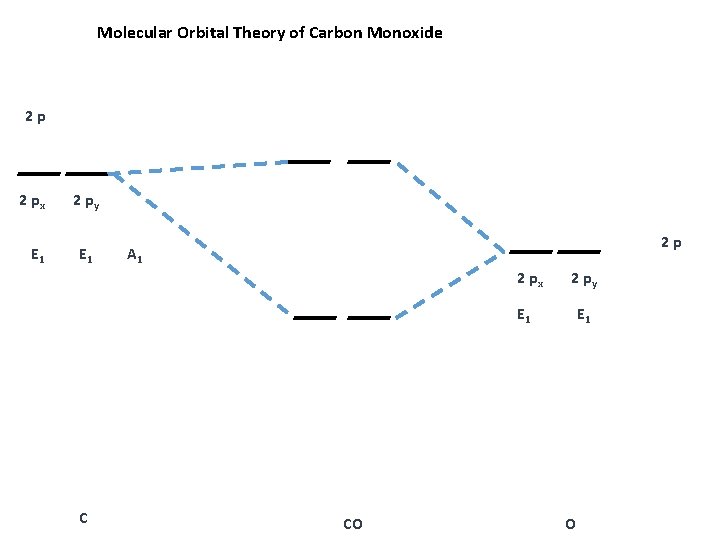
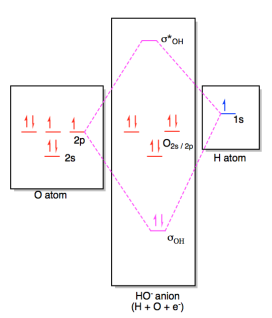
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Molecular Orbital Diagram for the HF Molecule. Interaction occurs between the 1s orbital on hydrogen and the 2p orbital in fluorine causing the formation of a sigma-bonding and a sigma-antibonding molecular orbital, as shown below. Figure 1: Molecular orbitals of HF. (CC BY-SA-NC 2.0 UK: England & Wales License; Nick Greeves).
hybridization and molecular orbital mo theory molecular shapes based on valence electrons lewis dot structures and electron repulsions •molecular orbital theory mo - a molecule is formed by the overlap of atomic orbitals to form molecular orbitals electrons are then distributed into mos a molecule is a collection of nuclei with the orbitals ...
Answer (1 of 2): * As we see Boron has 3 electrons in its valence shell. * The compound BH3 formation by vbt is shown in below figure, * Now we see that in valence shell ofBH3 there are 6 electrons. * But generally speaking about compounds, they are stable when they attain octate configuratio...
From this I built A1 bonding and antibonding orbitals, A'2 bonding and antibonding orbitals, 1 A'2 and 2 E''2 non bonding orbitals. I'm now stuck because I have 4 E' on the F side and only 2 E' on the B side. I found the following diagram for BF 3 online but it doesn't generate the E' anti bonding and also doesn't generate enough molecular ...
field molecular orbital (SCFMO) wave functions of H3N-BH3 and OC-BH3 complexes. Calculations and Results The wav efunctions for the ground states of BH3. NH3. CO. H3N-BH3' OC-BH3 have been calculated by the well known CNDO/2 formalism of Pople and coworkersll. 51'0 basis sets have been used and the core orbitals of C. B, N, 0 atoms have been ...
to join our neet/iit jee/iit-jam/csir-net/iit-gate/du/bhu/rpsc online regular/crash course please download the app and get registered there...app link-http:/...
Molecular Orbitals for BF3. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on a molecular orbital circle in the energy level correlation diagram shown The energy level diagram may be displayed with or without the group theory symbols and character table: the models accessed by clicking are the same.
Molecular orbital diagram for BF3 - Chemistry Stack Exchang . View Notes - 2_MO Diagrams with LGOs_BH3_KEY from CHEM 312 at University of Washington. Names: _ _ CHEM 312 In-Class Activity #2 Generate an MO diagram to describe the bonding in BH3. (D3 ; Kaninchen Polyklonal BAX Antikörper BH3 Domain, N-Term für IHC, ELISA, WB.
Molecular Orbitals for Larger Molecules 1. Determine point group of molecule (if linear, use D2h and C2v instead of D∞h or C∞v) 2. Assign x, y, z coordinates (z axis is principal axis; if non-linear, y axes of outer atoms point to central atom)3. Find the characters of the reducible representationfor the combination of
The molecular orbital diagram of NH3 is presented in Figure 5 and will be elaborated in regards to its interactions. The s orbitals for the 3 hydrogens are used to set up the sigma and anti bonding combinations of N sp 3 orbitals and the H 1s orbitals. Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the ...
Γbasis is the reducible representation. The "basis" will be either the 2s, 2px, 2py, or 2pz orbitals of boron, or the 1s orbitals of hydrogen. So, we'll be running over six bases! Yowza. χIRREP ˆR is each number for a given row in the character table. For example, in Au you would use 1, 1, 1, 1, −1, −1, −1, and then −1.
4. Molecular orbital theory. Borane (BH3) has trigonal planar geometry and D3h point group symmetry. Derive a molecular orbital diagram shown below for borane by performing the same steps as above in problem #3: (1) Determine a Γ describing the three H 1s atomic orbitals, (2) factor this Γ into a linear combination of Γirr, and (3) complete the molecular orbital diagram exactly as described ...
An advanced molecular orbital diagram of beh2 beryllium hydride for the inorganic or physical chemistry student. Molecular orbitals and walsh diagram. Walsh correlation diagram is a plot of molecular orbital energy as a function of some systematic change in molecular geometry. Be has 2s and 2p orbitals and it is in the middle.
We observe that each fluorine has a 2S, 2px, 2py and 2pz. If we take the 2pz as being along the internuclear axis then we assign it with sigma symmetry, while the x and y p orbitals have pi symmetry. It is the 2pz that can interact with the Hydrogens 1S because it has the correct symmetry. It turns out that the symmetry of that orbital is ag.
BF3 is SP2 hybridization. For this molecule, It is SP2 because one π (pi) bond is required for the double bond between the Boron and only three σ bonds are formed per Boron atom. The atomic S - orbitals and P - orbitals in Boron outer shell mix to form three equivalent SP2 hybrid orbitals.
Organic chemistry 03: Bonding - atomic orbitals and molecular orbitals. Jan 30, 2015 • ericminikel • Cambridge, MA • chem-20 These are my notes from lecture 3 of Harvard's Chemistry 20: Organic Chemistry course, delivered by Dr. Ryan Spoering on January 30, 2015.
atoms (1s orbitals). That leaves two B sp3 hybrid orbitals, one of which contains an electron, one of which is empty. For each bridge therefore, one sp3 orbital from each of the B atoms combines (Figure 3) with the 1s orbital of the bridging H atom to form three new molecular orbitals (MOs) - as always, n atomic orbitals (AO) form n MOs. One ...
Molecular geometry is associated with the chemistry of vision, smell and odors, taste, drug reactions and enzyme controlled reactions to name a few. BH3 is non-polar. Molecular Geometry of BF3. 1 decade ago. 5) What is the Molecular Geometry (MG)? Examples of molecular weight computations: C[14]O[16]2, S[34]O[16]2.
Our goal is to apply the principles of quantum mechanics and electronic structure theory to address problems in physical, organic, inorganic, and biological chemistry. High performance computers are used to solve the complex equations describing the system of interest, yielding predictions of structures, bonding, energetics, reactivity, and other physical properties
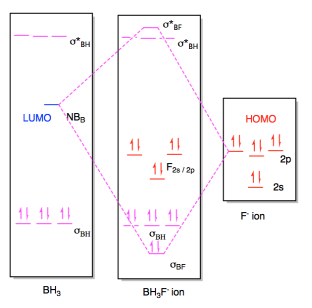
Expain acid and base interaction between Ph3P - BH3 You can use a molecular orbital to explain this. To simplify, you can consider the case between PH3 and BH3, rather than PPh3. The change from Ph to H has no major effect on the analysis. which specific molecular orbitals are involved in the Lewis acid-base interaction what are the ...
Recebido em 18/7/11; aceito em 14/2/12; publicado na web em 15/6/12 A simple, four-step method for better introducing undergraduate students to the fundamentals of molecular orbital (MO) theory of ...




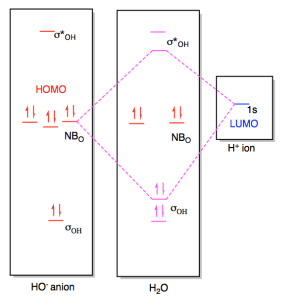
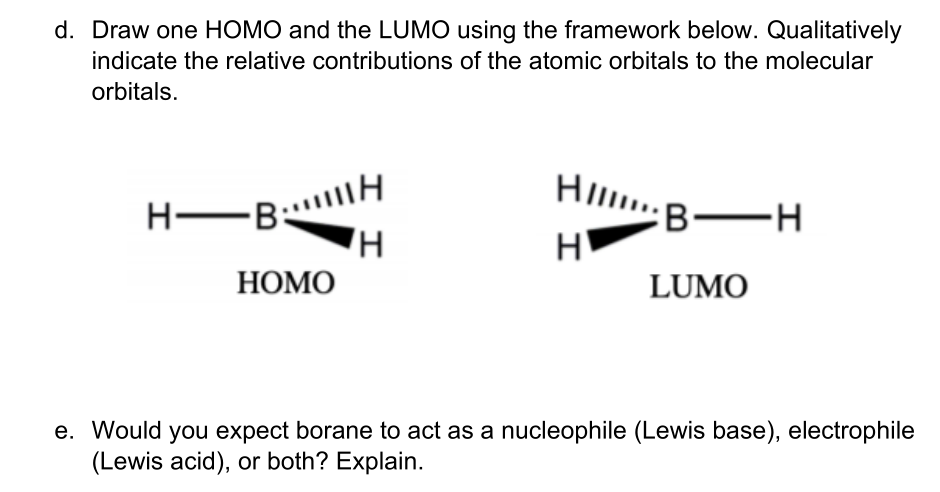

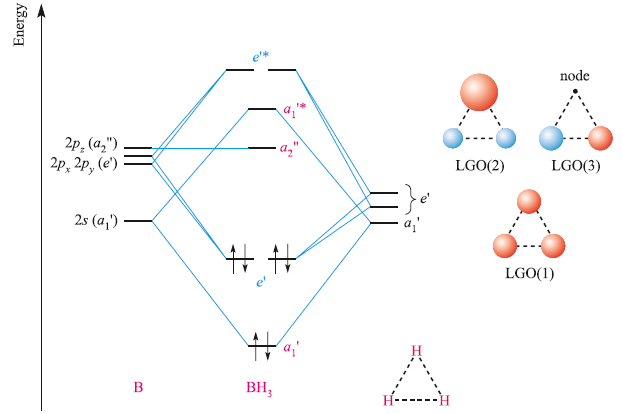
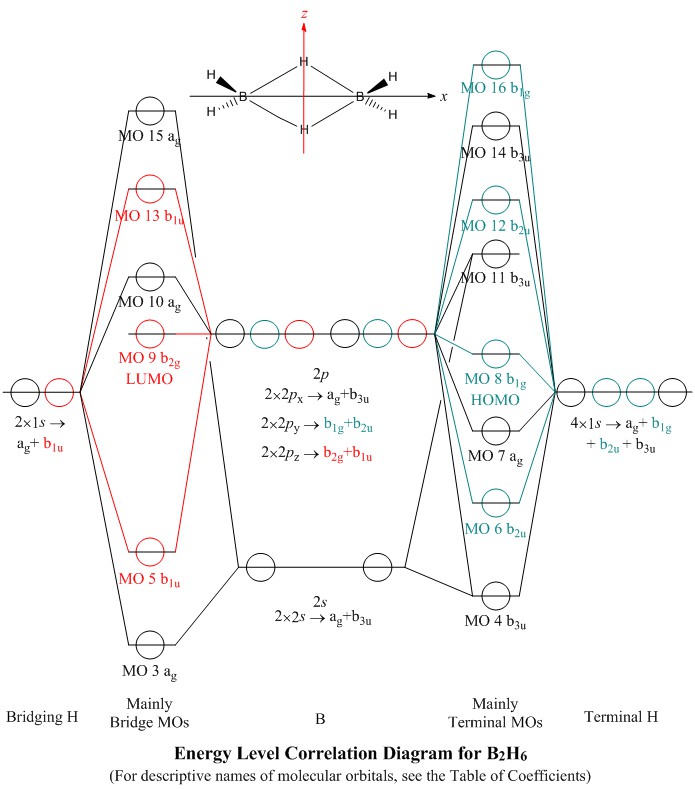












0 Response to "44 bh3 molecular orbital diagram"
Post a Comment