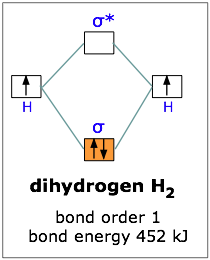
44 construct the molecular orbital diagram for h2 and then identify the bond order
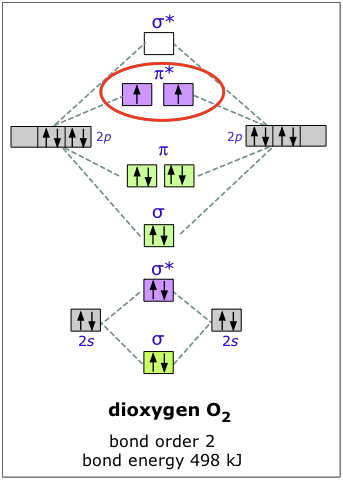
Construct the molecular orbital diagram for h2 and then identify the bond order. Click thin the blue boxes to add electrons. To determine the bond order of oxygen, we need to first draw its molecular orbital energy diagram, oxygen contains 12 valence electrons. So too in sigma to S to insignia to a star to insignia to pee, then four and the two P two P. S. And then the last to go into the P two P stars.
Bond order: 1s o 0.5 O 1.5 2 Atom Molecule Atom Click within the blue boxes to add electrons. The following is part of a molecular orbital energy-level diagram for MOs constructed from 1s atomic orbitals.
Construct the molecular orbital diagram for h2 and then identify the bond order
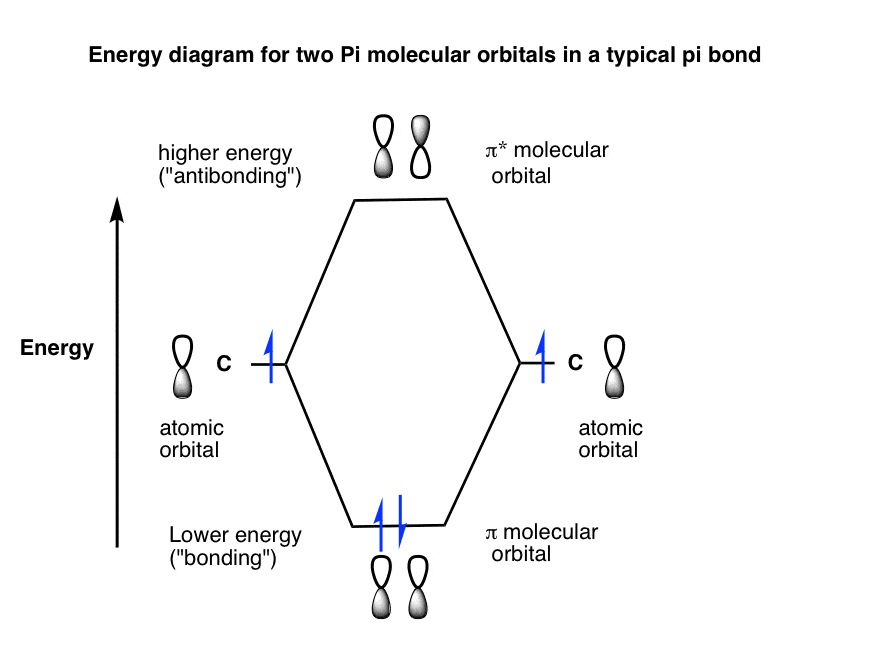
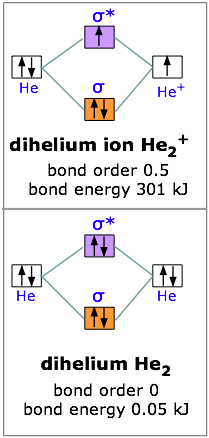
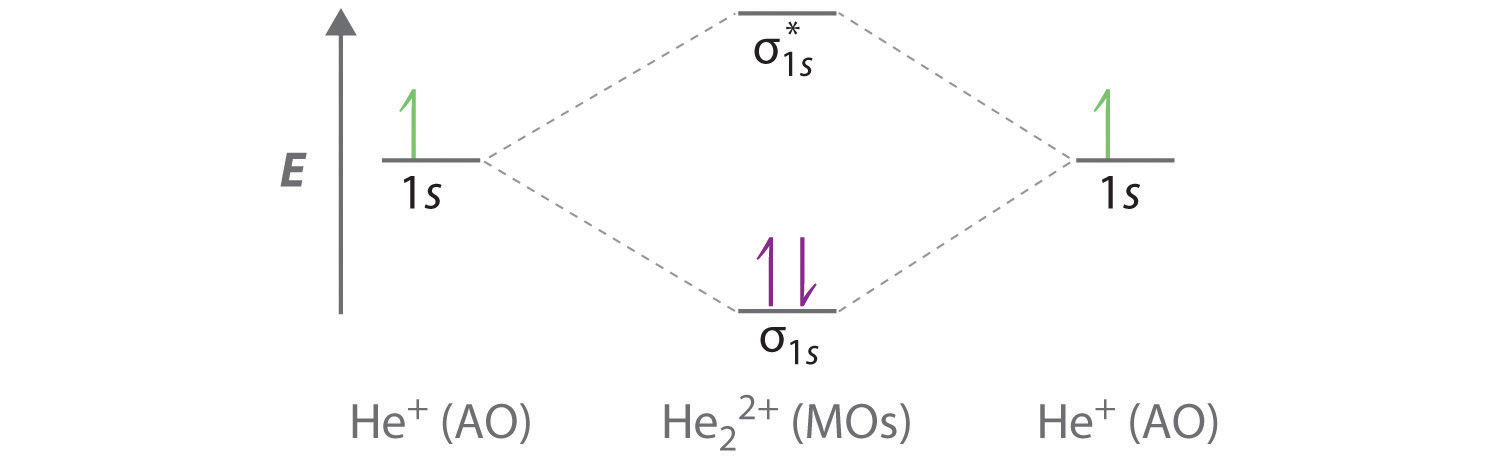
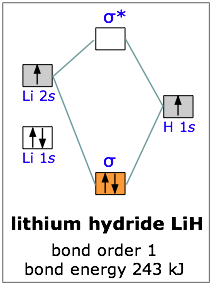
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. ...of the molecular orbital (MO) diagram like this: where sigma^"*" indicates an antibonding sigma (sigma) MO, and sigma is the bonding MO. At O_2 and past, the ordering of the sigma_(2p_z) and pi_(2p_(x"/"y)) switch. Filling the molecular orbitals: The first four electrons fill the sigma_(1s) and... Molecular orbital diagram for the molecule Heg is shown below. Bond order calculation: B.O=}(B.E - A.B.E) where, B.O=Bond order B.E=Bonding electrons A.B.E=Anti - bonding electrons Answer Bond order and the molecular orbital of the diagram is given below. bond order Energy...
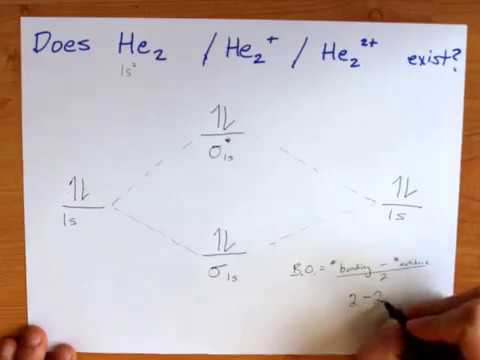
Construct the molecular orbital diagram for h2 and then identify the bond order. Molecular Orbital Theory Boundless Chemistry. Solved Construct The Molecular Orbital Diagram For He2 An. Chapter 9 Molecular Geometry And Bonding Theories Ppt Download. Chemical Bonding Electrochemistry Lower Chemical Bonding. Molecular Orbital A Molecule In Which All The... Answer : According to the molecular orbital theory, the general molecular orbital configuration will be, As there are 7 electrons present in nitrogen. The bond order of is, 3. The molecular orbital diagram of are shown below. laminiaduo7 and 94 more users found this answer helpful. Calculate bond order and determine magnetism summarize your findings for each molecule by determining if this molecule would exist and writ... 1 can bond order be a negative value. Discussed in this video are. Construct the molecular orbital diagram for he2 and then identify the bond A molecular orbital explicitly describes the spatial distribution of a single energy level diagrams he2 has bond order 0 2 22 0 and we can make h. Click...
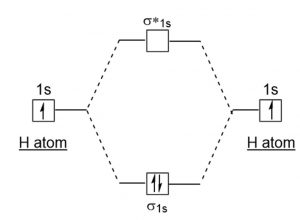
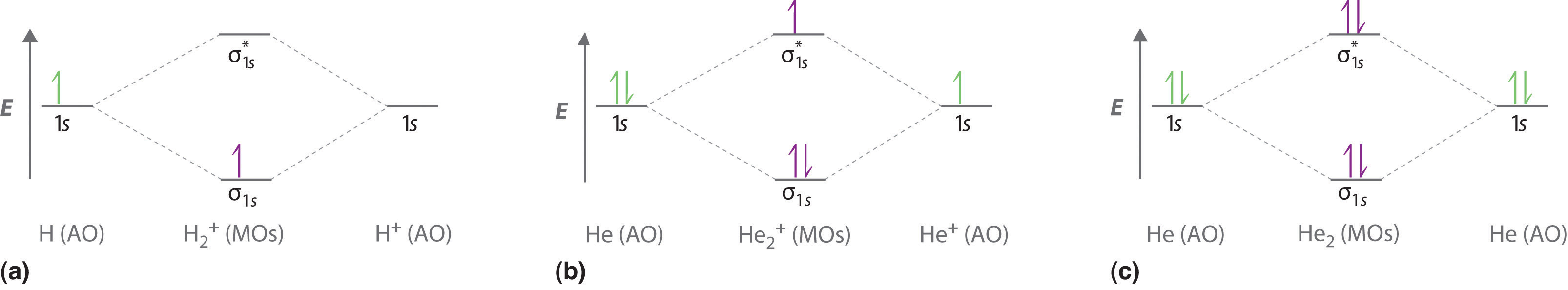
The molecular orbital energy level diagrams for H2, H2. +, H2. - and O2 H2. - will be longer. Both have bond order of , but H2. Indicate the lowest energy electron excitation in this ion by identifying the initial and.Show transcribed image text Construct the molecular orbital diagram for H2 and... Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between The next step in constructing an MO diagram is filling the newly formed molecular orbitals with electrons. Show transcribed image text construct the molecular orbital diagram for h2 and then identify the bond order. Ask for details. Log in join now 1. Click within the blue boxes to add Molecular Orbital Theory Boundless Chemistry. Energy Level Diagram For Molecular Orbitals Chemical Bonding And. Molecular orbital theory involves solving (approximately) the Schrödinger equation for the electrons We can then allow these wavefunctions to interfere constructively and destructively as we bring the Note that the bonding orbital in the MO diagram of H2 is stabilized by an energy β/1+S and the...
The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear If the z axis is identified with the internuclear axis, the 2s and 2pz orbitals on each atom all have cylindrical symmetry around the axis and hence... Since 1s shell of bonding orbital can accommodate only two electrons. So, next one electron will go into 1s shell of anti-bonding orbital. What would be the minimum extent of reaction p required to synthesize poly(ethylene terepthalate) to a number average molecular weight of 100 kg/mol. Molecular orbital diagram for nitrogen gas n2 use aufbau and hund to fill with 10 valence electrons you get sigma2s2sigma2s2pi2p4sigma2p2. Draw the lewis structure of pf 3a how many share. How to draw mo diagrams for h2 h2 he2 he22 indicate the bond order for each of the. Science. Chemistry Q&A Library Construct the molecular orbital diagram for H2. A: Given,The mass of CH3OH = 14.6 gAnd the mass of H2O = 184 gWe know that, the molar mass for CH3OH = ... question_answer. Q: what is the half life of an isotope that decays to 25% of its original activity in 68.9...
Since 1s shell of bonding orbital can accommodate only two electrons. So, next one electron will go into 1 s shell of anti-bonding orbital. - Bond order = (Bonding electrons - antibonding electrons) / 2.
In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons. Show transcribed image text construct the molecular orbital diagram for h2 and then identify the bond order. The bond order of a diatomic molecule is...
Molecular Orbital Diagram for Carbon Dimer (C2).Fill from the bottom up, with 8 electrons total.Bonding Order is 2, and it is Diamagnetic.sigma2s(2),sigma2s...
These candidate molecules are then carefully tested to determine side effects, how effectively they can be Figure 5.34 This is the molecular orbital diagram for the homonuclear diatomic. In the molecular orbital model, an electron contributes to a bonding interaction if it occupies a bonding...
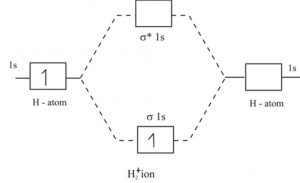
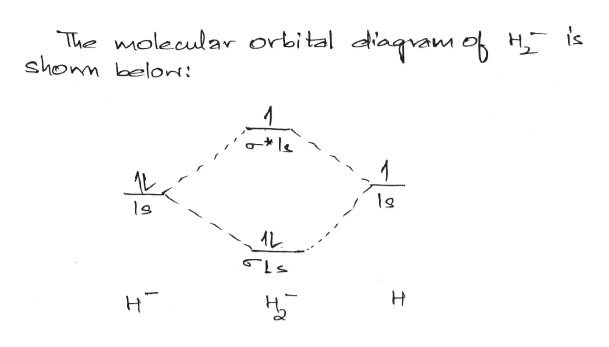
Transcribed image text : Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: 0 0.5 1 1.5 2 Click within the blue boxes to add electrons.
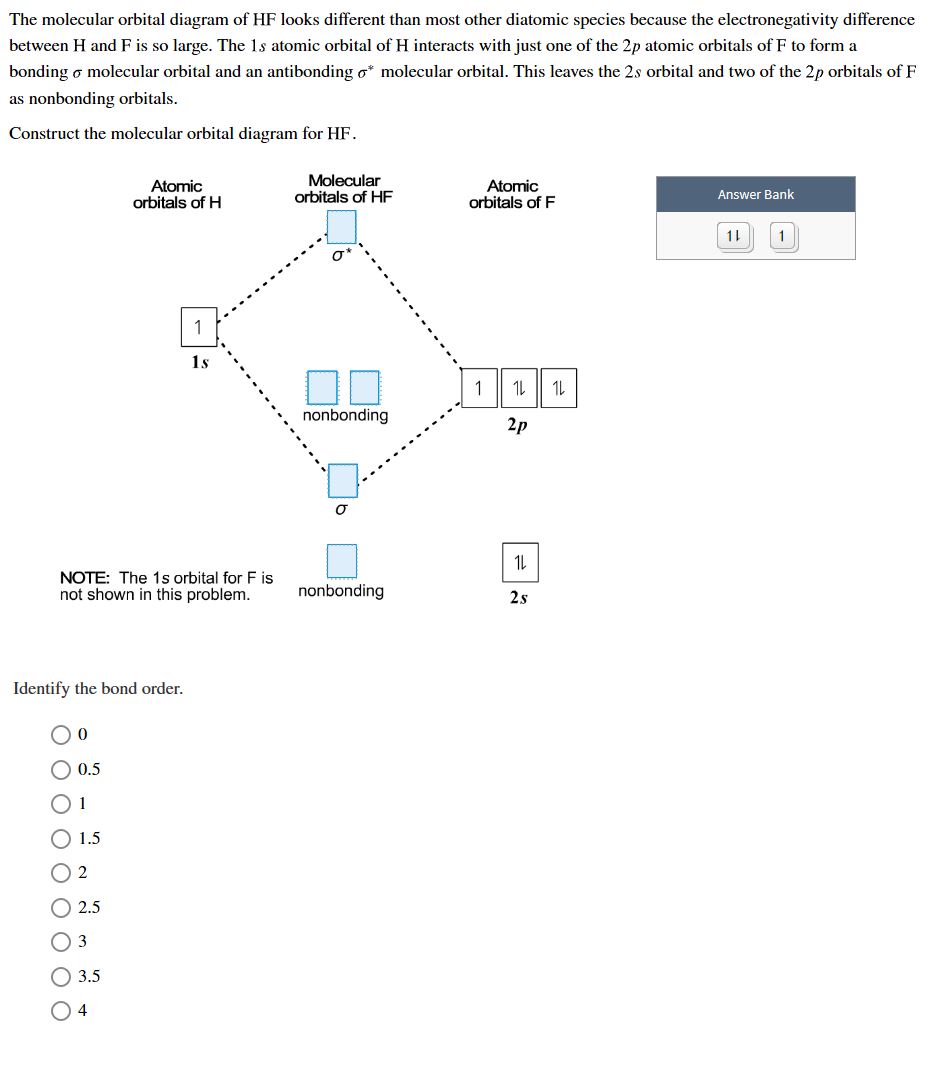
Draw the molecular orbital diagram of N_2 molecule and write its molecular orbital configuration. Ca... Molecular orbital theory,bond order of How to calculate bond order from molecular orbital diagram? Use the drawing of a molecular orbital energy diagram for ClF (Assume that the ?p...
These candidate molecules are then carefully tested to determine side effects, how effectively they can be Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the In the molecular orbital model, an electron contributes to a bonding interaction if it occupies a...
C would this ion exist. Bonding mos antibonding mos and bond order. Introduction To Molecular Orbital Theory A d...
Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the geometry of the molecule to which they are applied. Molecular orbitals are obtained by combining the atomic orbitals on the atoms in the molecule. Consider the H2 molecule, for example.
The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
Answer to construct the molecular orbital diagram for h2 and then identify the bond order. Discussed in this video are. According to molecular orbital mo theory two atoms mix their orbitals to form one that is spread out over both atoms.
Molecular Orbital Theory. Bonding and Antibonding Molecular Orbitals. In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of The energies involved in the molecule's various motions can then be broken down into three categories
Molecular orbital diagram for the molecule Heg is shown below. Bond order calculation: B.O=}(B.E - A.B.E) where, B.O=Bond order B.E=Bonding electrons A.B.E=Anti - bonding electrons Answer Bond order and the molecular orbital of the diagram is given below. bond order Energy...
...of the molecular orbital (MO) diagram like this: where sigma^"*" indicates an antibonding sigma (sigma) MO, and sigma is the bonding MO. At O_2 and past, the ordering of the sigma_(2p_z) and pi_(2p_(x"/"y)) switch. Filling the molecular orbitals: The first four electrons fill the sigma_(1s) and...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.





















0 Response to "44 construct the molecular orbital diagram for h2 and then identify the bond order"
Post a Comment