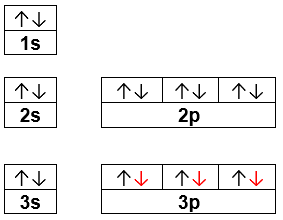
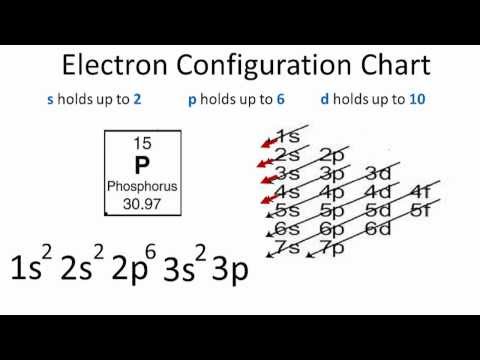
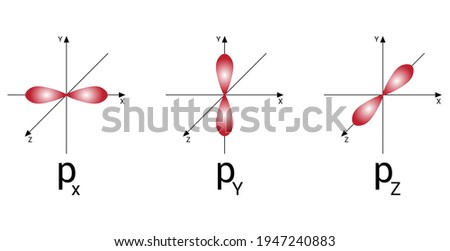
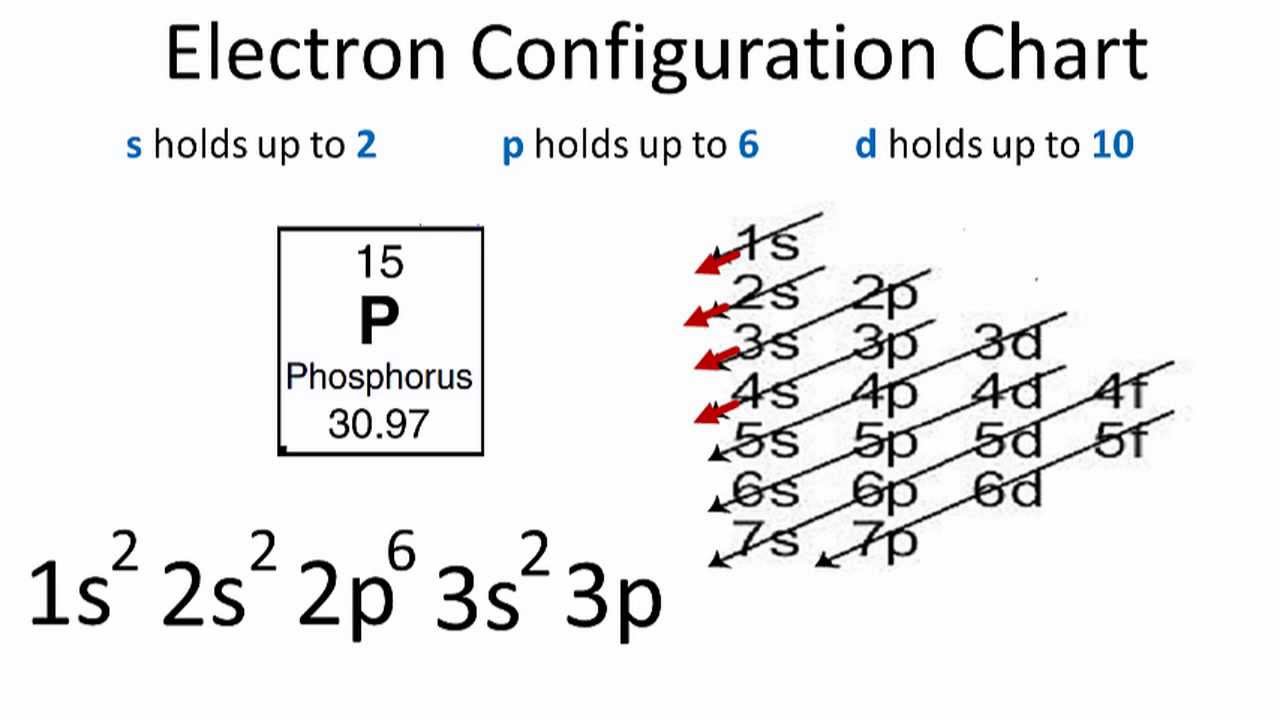
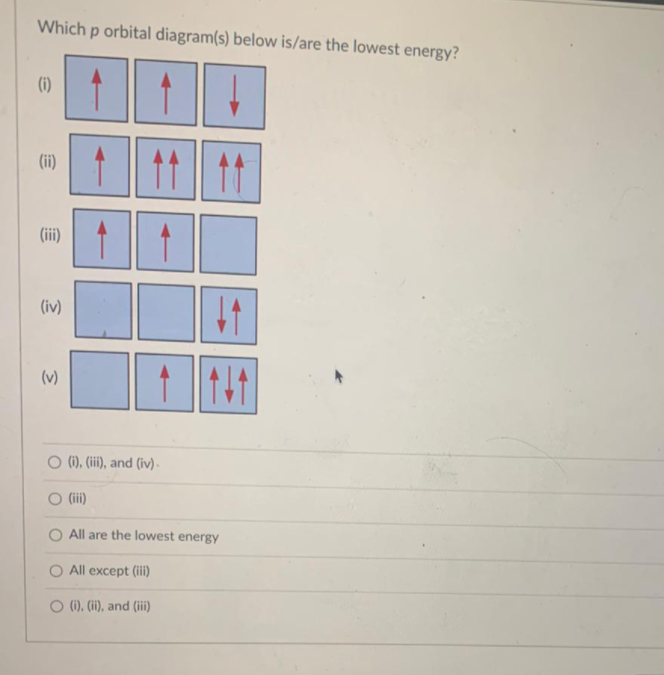
44 orbital diagram for p
The highest occupied orbital (HOMO) is. , but this diagram does not show it as occupied because it is using electrons for e.g. . (The reason that. is pyramidal is because its HOMO is lower in energy than it would be in a planar geometry.) This diagram does not use hybrid orbitals...
Figure 19.14 Molecular orbital diagram for an octahedral complex of a first series transition metal (only a interactions are considered in this simplified diagram). Figure B A qualitative molecular orbital diagram for ferrocene. The subscripts g and u refer to the parity of the orbitals g (German gerade...
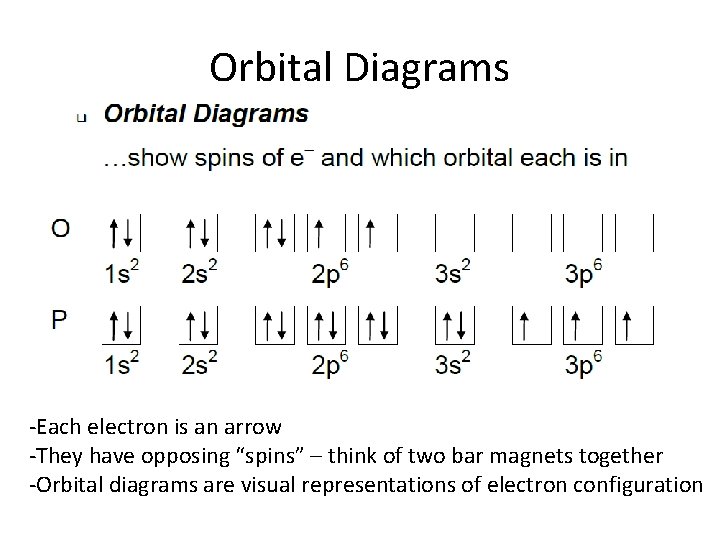
Electron configuration of oxygen atom through orbital diagram. Atomic energy levels are subdivided into sub-energy levels. These sub-energy levels are called orbital. The sub energy levels are expressed by ‘l’. The value of ‘l’ is from 0 to (n – …
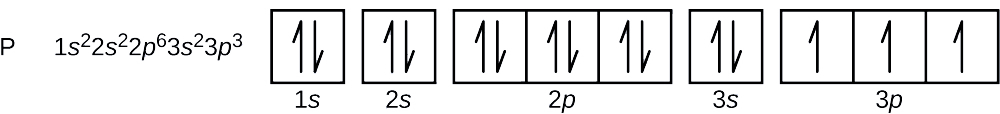
Orbital diagram for p
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram...
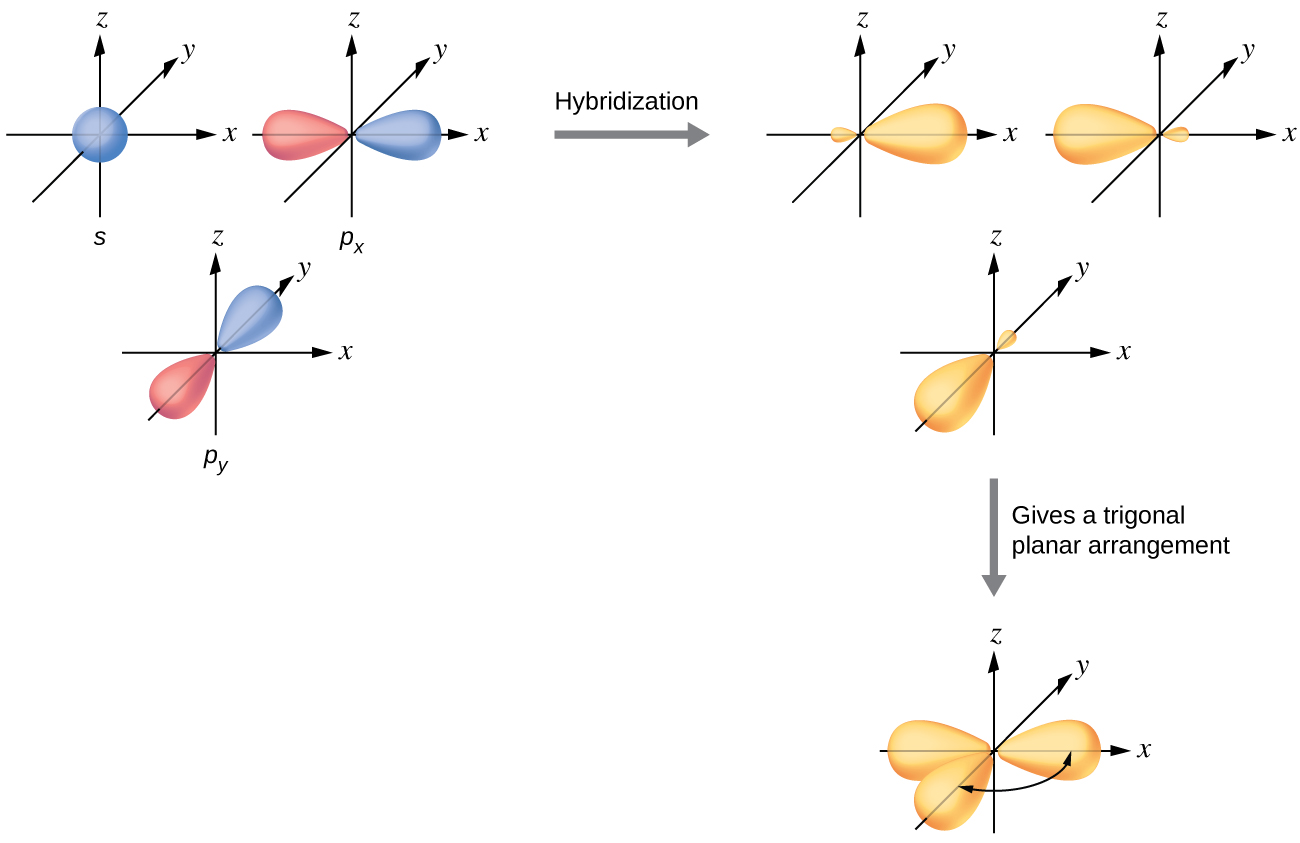
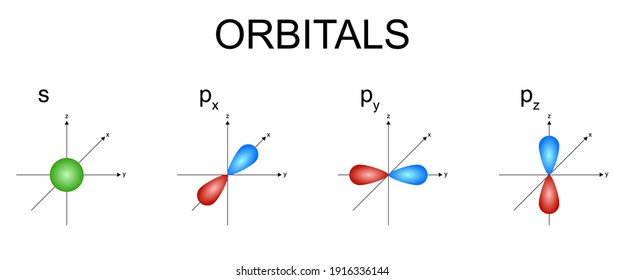
While Lewis diagrams and energy level structures can show connectivity and energy relationships of mol-ecules, they do not show the shape of the molecules. The picture above shows the spherically symmetric 1s orbital in the 'green' phase. Sometimes it is more con-venient not to show the phase, in...
[](/# Auto Sync Start) #### Quick Links[](https://www.flickr.com/photos/spacex/51369631902/) [^(NERDLE CAM)](https://www.youtube.com/watch?v=_HZCh2eGWEI) ^| [^(LAB CAM)](https://www.youtube.com/watch?v=HGb28t5TWtc&t=0s) ^| [^(SAPPHIRE CAM)](https://www.youtube.com/watch?v=7OKb9Rc-etw&t=0s) ^| [^(SENTINEL CAM)](https://www.youtube.com/watch?v=DC_jKgtoZOE&t=0s) ^| [^(ROVER CAM)](https://youtu.be/5HpgJJ1FwTc) ^| [^(PLEX CAM)](https://www.youtube.com/watch?v=FcjHfaCEddA&t=0s) ^| [...
Orbital diagram for p.
How then do you draw out the molecular orbital diagram for the new bond formed if you use the 2 electrons and place them in the antibonding orbital of H-Br bond? If these 2 electrons are in that antibonding orbital, what is going into the bonding orbital to form the bond between nucleophile and hydrogen atom?
In my review sheet for bonding it says "Draw the orbital diagram for one of the fluorine atoms in a molecule of XeF4.". Do i just draw the diagram for the un-bonded Fluorine atom? Is it the same thing as drawing the diagram for a bonded Fluorine atom? If not, how would i write/draw the bonded Fluorine atom in XeF4?
The last orbital of fluorine is ‘p’ and unpaired electrons exist in its last p-orbital. And, p-orbital has five electrons. An electron exists in the last (2p z) sub-orbital. Whose spin is one-way. So, The fluorine atom supports the Hund principle. Determination of group and period through the electron configuration
• The following slide illustrates the relative energies of the molecular orbitals compared to the original atomic orbitals. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the H2...
The next element has two electrons and the second electron fills the 1s orbital because there are only two possible values for the spin quan...
Drawing orbital diagrams gives information not only about the orbitals that are/have been filled but also about the number of unpaired electrons. Orbital diagrams can be cumbersome!!.
I'm not sure what my Professor means when he says "Draw the orbital diagram for the hybridized Xe atom in a molecule of XeF4." and "Draw the orbital diagram for one of the fluorine atoms in a molecule of XeF4." I think i'm just confused by the wording because it seems easy but i have no idea. Could you guys explain this to me in another way? Thank you.
1 hours ago orbital diagram (orbital box diagram) : 1s box has 2 arrows (as per helium above), 2s box has 2 arrows as per boron above, but now we see that there are 3 orbitals that make up the p-subshell (p x, p y, p z), into which we need to place 2 arrows. So, we apply Hund's Rule so that we maximise...
The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to...
Draw a molecular orbital diagram for He2. Use this diagram and bond order calculations to explain if He2 is more or less stable than the ions He2+ and He2−. I have done He2 and He2+ and the bond order calculations for both. I just wanted to know why you cant do the same for He2-
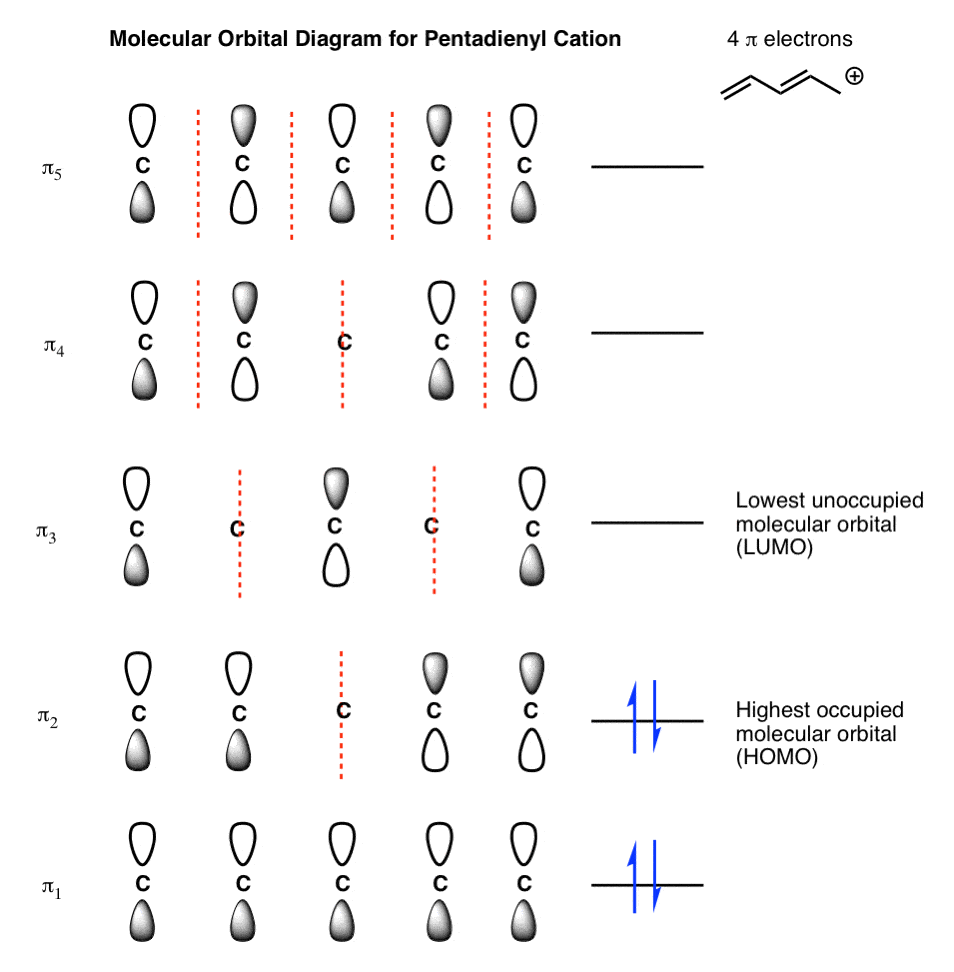
Hi! I'm doing some inorganic chem homework and I drew the molecular orbital diagrams for a few molecules and now I have to assign and draw the HOMO and LUMO. Does the HOMO have to be the highest totally occupied orbital or can it just be partially filled? Does the LUMO have to be totally unoccupied or can it just be partially unoccupied? Thanks in advance!
Molecular orbital (MO) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wavefunctions. Application: Computational Chemistry in Drug Design. Molecular Orbital Energy Diagrams. Bond Order. Bonding in Diatomic Molecules.
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration.
Oct 17, 2018 · The $\mathrm{s}$-$\mathrm{p}$ interaction is the bonding interaction between the $\mathrm{2s}$ orbital of one atom and the $\mathrm{2p_{z}}$ orbital of another atom which (among other things) increases the energy of the $\mathrm.Molecular orbitals of diatomic molecules.Molecular orbital diagram - Wikipedia
When we draw the Lewis structure, the best structure is the one where S has the negative formal charge and each fluorine is singly bonded to the sulfur. So in my tutorial question, we are asked to draw the valence orbital diagram for F, S, S-, S*- and S hyb. So for F and S it's just the normal case. But for S-, isn't this assuming that sulfur has the negative ionic charge? I thought all it has is a negative formal charge where the formal charge does not indicate the presence of a negative ioni...
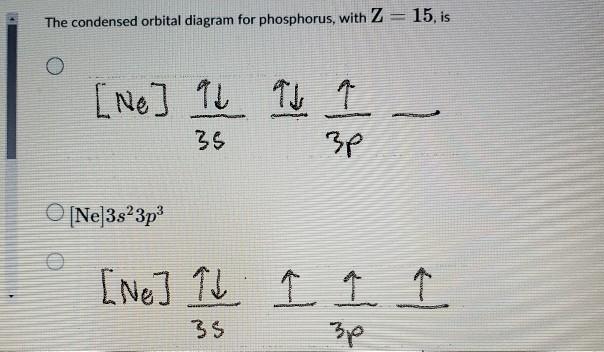
What would the orbital diagram for these molecules look like if they had this many electrons? ​ I'd guessed that phosphorus would have 3s(2), 3p(3), and 3d(5). The half-filled p and d orbitals would offer stability to explain why phosphorus can sometimes make 5 bonds. Alternatively I was considering 3s(2), 3p(6), and 4s(2). ​ I'd guessed that sulfur would have 3s(2), 3p(3), 3d(5), and 4s(2). Again, the half-filled p and d orbitals would offer stability. An electron from...
**This thread is no longer being updated, and has been replaced by:** # [Starship Development Thread #27](https://www.reddit.com/r/spacex/comments/qpup8z/starship_development_thread_27/) [](/# Auto Sync Start) #### Quick Links[](https://www.flickr.com/photos/spacex/51369631902/) [^(NERDLE CAM)](https://www.youtube.com/watch?v=_HZCh2eGWEI) ^| [^(LAB CAM)](https://www.youtube.com/watch?v=HGb28t5TWtc&t=0s) ^| [^(SAPPHIRE CAM)](https://www.youtube.com/watch?v=7OKb9Rc-etw&t=0s) ^| [^(SEN...
I have a quick question regarding the molecular orbital theory. We are doing cycloadditions right now, so we are using the HOMO/LUMO of dienes and dienophiles. I was wondering how to distinguish how many arrows a compound will have in the molecular orbital diagram? for instance 1,3-butadiene has 4 arrows in the diagram. Thanks
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in each orbital. Refer to the related link to see an illustration of an orbital diagram for aluminum.
- MO diagrams for Transition metal complexes. • For chemical reaction the HOMO (Highest Occupied Molecular Orbital) and the LUMO (Lowest unoccupied Molecular Orbital) are the most important.
At the moment I'm learning about molecular orbital diagrams for homonuclear molecules, namely: B2, C2, N2, O2, F2, and Ne2. I understand that the energy of the 2p sigma bond is at a higher level for B2, C2, and N2, leading to the 2p sigma bond and the 2p pi bond switching places in the MO diagram (with 2p pi bond appearing under 2p sigma bond) for B, C, and N but not for O, F, or Ne. My lectures state that this is due to s and p mixing and my textbook states that it is due to electron repulsion ...
Orbital Filling Diagram For Boron. How Many Orbitals Are In The 4p Subshell. Hund U2019s Rule And Orbital Filling Diagrams. Orbital Diagram For Neon U2014 Untpikapps. Ppt. Solved Entproblemid. Does The Electron Configuration Ar 4s23d5. Unit3presentation.
**This thread is no longer being updated, and has been replaced by:** # [Starship Development Thread #28](https://www.reddit.com/r/spacex/comments/rc8jw2/starship_development_thread_28/) [](/# Auto Sync Start) #### Quick Links[](https://www.flickr.com/photos/spacex/51369631902/) [^(NERDLE CAM)](https://www.youtube.com/watch?v=_HZCh2eGWEI) ^| [^(LAB CAM)](https://www.youtube.com/watch?v=HGb28t5TWtc&t=0s) ^| [^(SAPPHIRE CAM)](https://www.youtube.com/watch?v=7OKb9Rc-etw&t=0s) ^| [^(SEN...
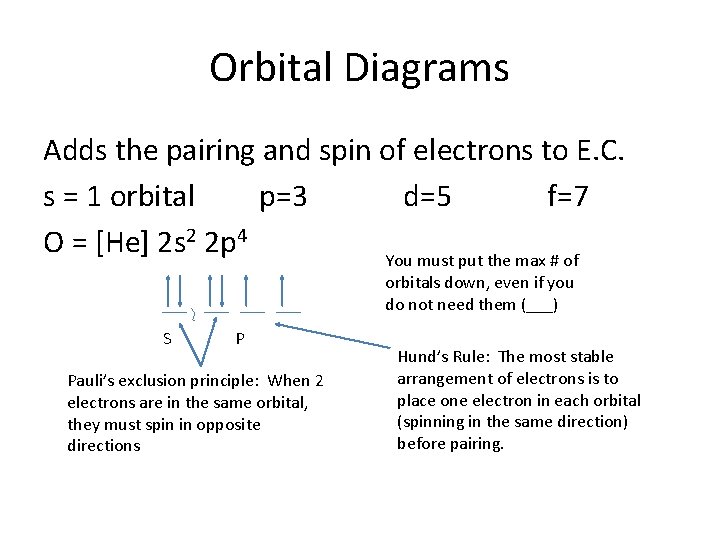
Start studying Orbital Diagrams. Learn vocabulary, terms and more with flashcards, games and other study tools. orbital diagram for sodium. Aufbau Principle. Electrons enter orbitals with the least amount of energy first.
I am dealing with several questions that are basically all the same but the basic form of the questions are asking to build a molecular orbital diagram for an unspecified Metal-Ligand complex. ie- Using a group theory approach, develop a molecular orbital diagram for a trigonal pyramidal complex [ML4]. Consider only sigma-orbitals. Start by finding the point group (C3v), then irreducible representations [2(A1)+E], and finally draw a qualitative molecular orbital diagram. Include pictures of th...
[Arrow pushing mechanism for nucleophilic addition.](https://imgur.com/a/hZxwD45) So I know how to draw the nucleophilic addition mechanism and I also know the molecular orbital diagram for the carbonyl group: [https://imgur.com/a/EzB4ZKw](https://imgur.com/a/EzB4ZKw). The empty LUMO is closer to carbon and therefore the incoming nucleophile (hydride in this instance) attacks the carbon instead of oxygen. This fills the 3pi\* orbital which destructively interferes with the 3pi bonding orbital. T...
Molecular orbital energy level diagrams for these second-row heteronuclear diatomics can be drawn rather easily by modifying the homonuclear pattern Solution: The molecular orbital diagram is similar to that for O2, shown in Figure 2-4. The peroxide ion has two more antibonding electrons than neutral...
Obtain the molecular orbital diagram for a homonuclear diatomic ion by adding or subtracting electrons from the diagram for the neutral molecule. We draw a molecular orbital energy diagram similar to that shown in Figure 8.37. Each oxygen atom contributes six electrons, so the diagram...
Cross posted from chem help so forgive me if for some reason you’re seeing this twice now. I’m working through a problem set for one of my classes and so far it’s had me make the SALCs and molecular orbital diagram for NH3. Now it’s asking me to go from constructing the MO diagram to identifying the ground electronic state of the molecule. I’m guessing what I need is some kind of wave function to describe the population of each orbital in the ground state. But I’m not sure how to get there fro...
Also, using the Molecular orbital diagram of CN-we can also find its bond order which helps us to predict its bond length and stability as well. Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5.
View Orbital_Diagram_Practice from SCIENCE S760 at Villa Park High. Orbital Diagram Worksheet A. Draw an orbital diagram for each of the following The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in...
I don't understand how to use the Afbau principle. At all. I have no clue. I'm assuming this is probably why I'm absolutely lost when drawing an orbital diagram, lol. The question is "write the full electron configuration and draw the orbital diagram for an atom of titanium in its ground state". I have zero clue on where to even start. I've been trying to understand orbitals for the better part of the morning, and I still have no clue what I'm doing, lmao. Please help me? If you have any resour...
I'm working on a lab for dynamical systems that involves making an orbit diagram for fifty values of c for the quadratic family x^2 + c and then sketching the points on the orbit diagram on a piece of paper. What I want to do is somehow make a cumulative orbit diagram with every point I enter. I know nothing about Mathematica, so I'm not sure how easy or possible this is. I'll link the notebook I'm working with, I'd appreciate any suggestions or help. http://math-cs.cns.uni.edu/~ostapyuk/Orbit...
I’m working through a problem set for one of my classes and so far it’s had me make the SALCs and molecular orbital diagram for NH3. Now it’s asking me to go from constructing the MO diagram to identifying the ground electronic state of the molecule. I’m guessing what I need is some kind of wave function to describe the population of each orbital in the ground state. But I’m not sure how to get there from where I am. Any tips are appreciated. I also have to work out which transitions from the ...
I'm trying to build a molecular orbital diagram for BF3 and I'm running into problems with irreducible representations on the F side. 2s for B has an irreducible representation of A1. 2p for B has an irreducible representation of E' and A''2. 2s for F considered non bonding.
Do you want to know more information about Platinum Orbital Diagram? In this article, you will find many highest-rated Platinum Orbital Diagram pictures we unearthed on the internet. We identified it from reliable sources that discuss Platinum Orbital Diagram.
In the Electron Configurations of Main Group Elements lesson, you learned a little bit about valence electrons. You saw how the number and type of valence electrons are important in determining the chemical properties of a particular element.
For elements 1-36, there are two exceptions to the filling order as predicted from the periodic table. Draw the atomic orbital diagrams for the two exceptions and indicate how many unpaired electrons are present. I'm not even sure where to start to be honest...
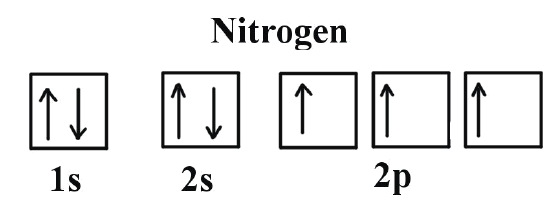
Orbital Diagrams for Period 2 Elements. The Aufbau Principle tells us that the first energy level (K shell) containing the 1s orbital was completed The orbital diagram for each Period 2 element will begin with a box occupied by 2 arrows (one up, one down) representing the completed 1s orbital (1s2).
An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. (Video) Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice Problems. By: The Organic Chemistry Tutor.
"Which substance would you expect to be the most magnetic: Fe, Fe+2, Fe+3? Draw the orbital diagrams for each species and use this information in your explanation of your answer. No explanation = no credit." So I was able to draw the orbital notation for each ion. I also recall that when dealing with ions for transition metals, I remove from the s subshell first. I do not understand which is the most magnetic. They all have unpaired electron shells. Is it Fe^+3 , due to the fact that it has 5...
hey! I have a question: how do i draw a molecular orbital diagram for SO2? i only found examples for diatomic diagrams and im not sure how to do it if i have more then two atoms in the molecule.
Electronic configuration of the Cobalt atom in ascending order of orbital energies: 1s2 2s2 2p6 3s2 3p6 4s2 3d7. Below is the electronic diagram of the Cobalt atom. Distribution of electrons over energy levels in the Co atom 1-st level (K): 2 2-st level (L): 8 3-st level (M): 15 4-st level (N): 2.
I need to construct the molecular orbital diagram for the hypothetical species Li4, which has the following geometrical arrangement: https://preview.redd.it/npsjre5pch571.png?width=197&format=png&auto=webp&s=c2a7948c2efa04a975bee1db722838fae7482456 The first step is to identify the point symmetry group. In this particular case, we consider that there is only one axis of rotation of order four (actually, other symmetry elements can be observed, but this is a previous consi...
Feb 23, 2016 · The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: A few things to keep in mind here: The reason we show three p-orbitals is that p-orbitals come in groups of three.
To draw an orbital diagram for an uncharged atom, Step 1 Write the complete electron configuration for the atom. Orbital diagram A drawing that uses lines or squares to show the distribution of electrons in orbitals and arrows to show the relative spin of each electron.
Nov 21, 2018 · We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory.
Add these electrons to the atomic and molecular orbitals. There is one p orbital on boron but there is no adjacent atom with another p orbital. The latest update to chemdoodle v32 introduces a new toolbar the orbital toolbar. If you are not found for Molecular orbital diagram practice worksheet, simply look out our info below : Recent Posts. 1.
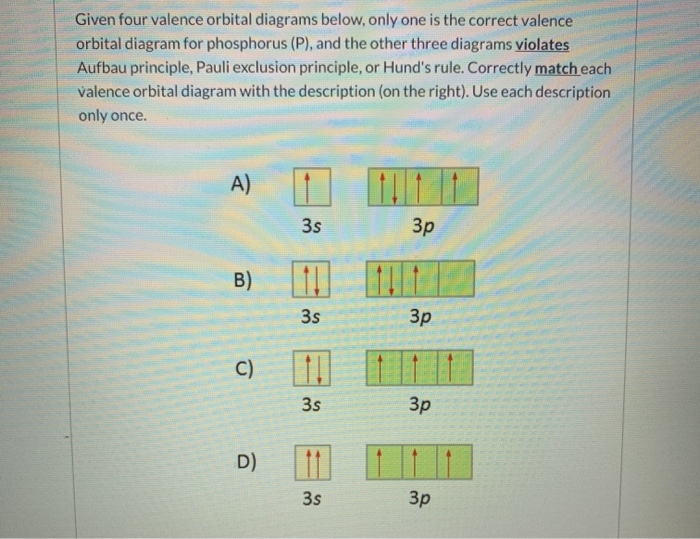
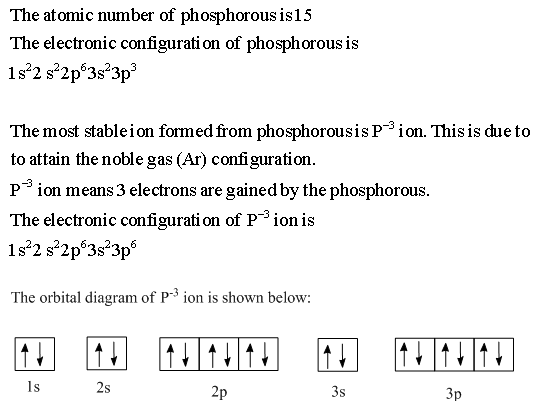
Orbital Diagram For Phosphorus. This makes it easier to understand and predict how atoms will interact to form chemical bonds. Help making the orbital filling diagram for electron configuration of phosphorus and iron. Build The Orbital Diagram For The Ion Most Likely Formed ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine …
Orbital Diagrams & Electron Configurations for Atoms and Ions Orbital diagrams represent the arrangement of electrons in orbitals. • boxes or lines represent each orbital • arrows within boxes represent the electrons • max two per box • opposite direction (represents opposite spin) Section 3.5...
Alright let's talk about orbital diagrams. Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest...
I have a homework problem asking me to construct the molecular orbital diagram for methylene chloride, and I am not too sure what to do next. I have determined that all of the orbitals transform as follow: C 2S=A1 C 2Pz=A1 C 2Py=B2 C 2Px=B1 2H 1S=A1+B2 2Cl 3S=A1+B1 2Cl 3Pz=A1+B1 2Cl 3Py=A2+B2 2Cl 3Px=A1+B1 My thoughts were to construct the MO for the CH2 side, then add the two chlorines from there. Let me know if you have any pointers. thank you























0 Response to "44 orbital diagram for p"
Post a Comment