40 energy diagram endothermic and exothermic reaction
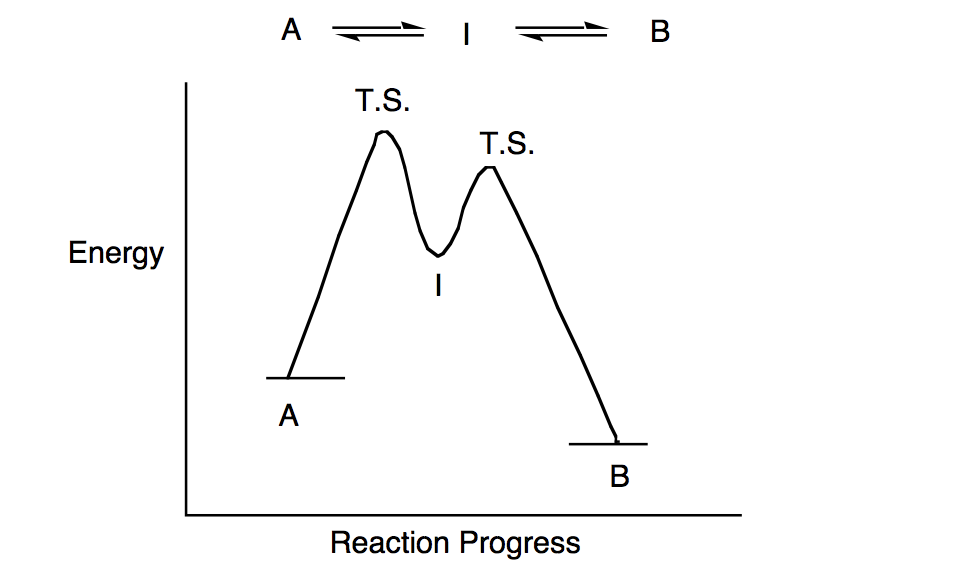
Consider, for example, a diagram that charts the energy change when a candle burns. Wax (C H ) combusts in the... energy and work. Using thermodynamics, you’ll learn how to calculate the precise amount of energy used or released by chemical reactions. Classifying a chemical reaction as exothermic or endothermic is simple. It... the reaction coordinate.(24) The phase diagram for NO/Pt(111) was generated by calculating the grand potential (Ω) at discrete coverages of 0.25, 0.50, 0.75, and 1.00 monolayer (ML). The grand potential is defined as where E NO/Pt is the total energy of the Pt(111) slab with N NO NO molecules adsorbed, E Pt is the total energy... preference, and top views of the minimum energy adsorption modes for... Reaction mechanisms for N–O bond...
Here we have investigated reaction pathways and relevant potential energy surfaces (PES) of reactions between Ti+ and TFA in the gas phase using the DFT method. Based on calculated results, we propose the most favorable reaction pathway in the reaction between Ti+ and TFA. CALCULATION METHODS▼ The molecular geometries and... 014 Copyright © 2012, The Korean Chemical Society Density Functional Theory Study of Competitive Reaction...

Energy diagram endothermic and exothermic reaction
Chemical Reactions Unit Miss E. Mac's Classic; Chemical Reactions Unit Miss E. Mac's Class Of; Chem I Unit 5 Reactions Chemical Reaction: a process in which one or more substances are converted into new substances with different physical and chemical properties Reactant: a substance that enters into a chemical reaction Product: A substance produces by a chemical reaction. the reaction written above exothermic or endothermic? Explain. The enthalpy change deals with breaking two mole of O-H bonds and the formation of 1 mole of O-O bonds and two moles of H-H bonds ( ). The sum of the... 7 kJ/mol which is an output (released) energy = 872.8 kJ/mol + 498.7 kJ/mol = 1371.5 kJ/mol. Total energy... Explore the 4 factors affecting rates of a chemical reaction and how they affect it. 9. Draw the energy diagram of an exothermic reaction showing: energy of reactants & products, uncatalyzed activation energy, catalyzed activation energy, transition state (activated complex), and energy of the reaction (heat of reaction) 10.
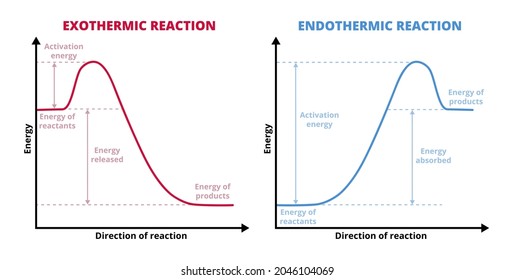
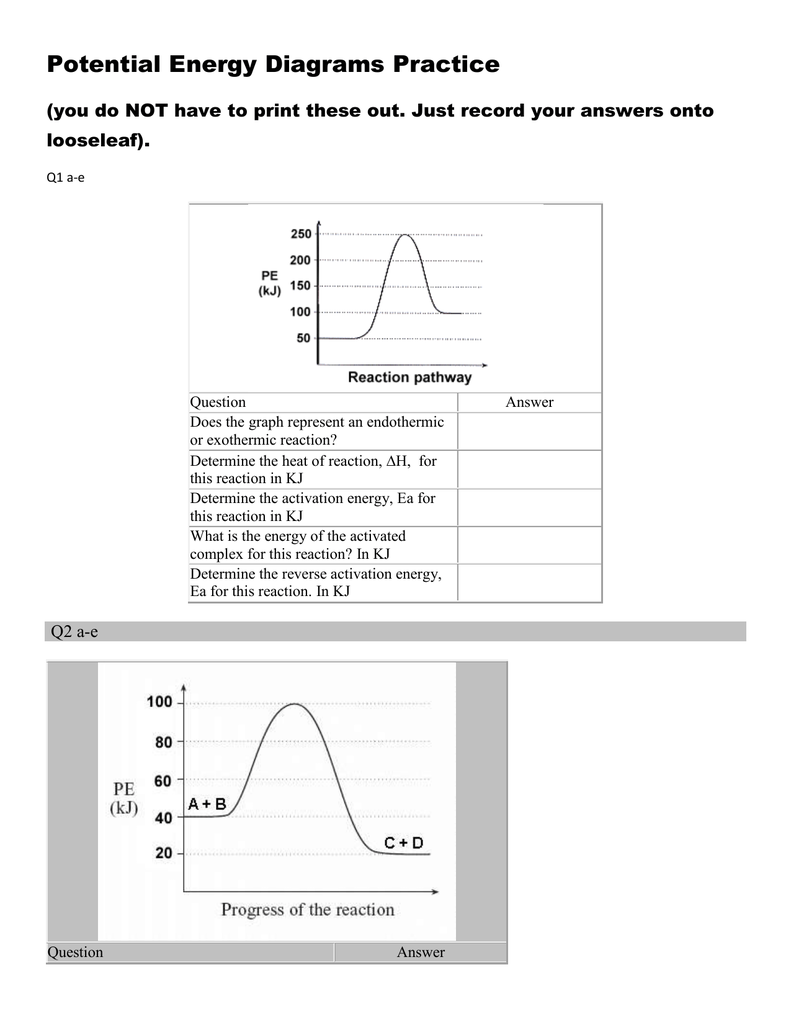
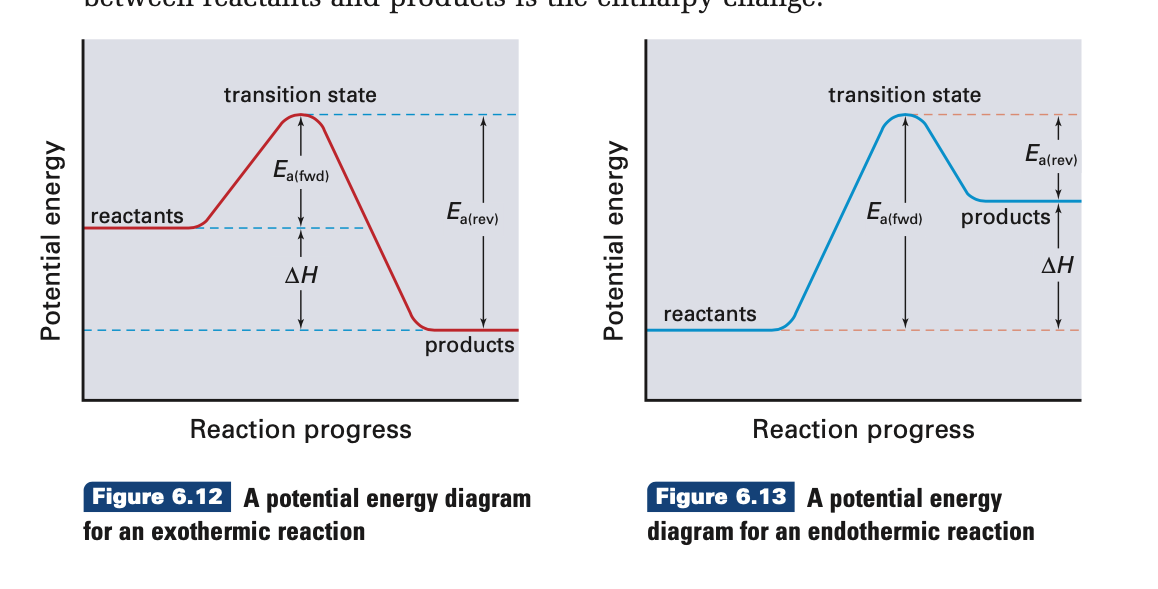
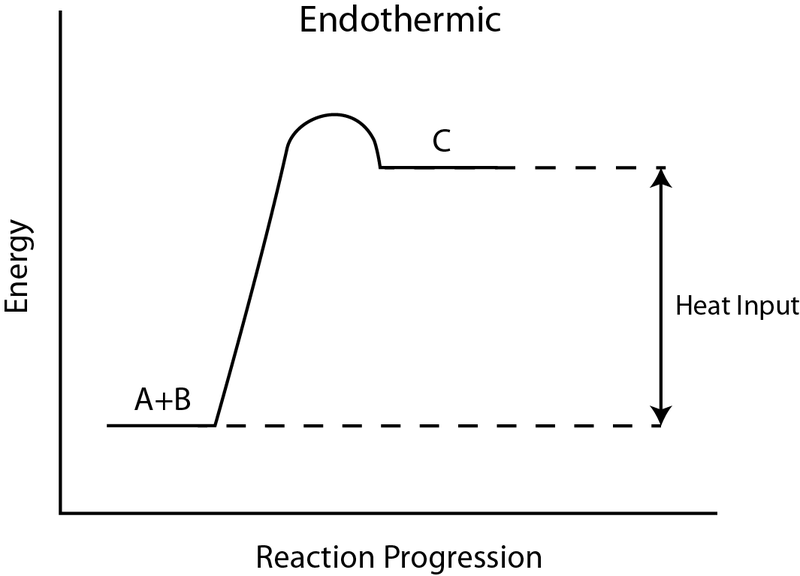
Energy diagram endothermic and exothermic reaction. 6. draw an energy diagram for a reaction. label the axis, pe of reactants = 350 kj mol, ea = 100 kj mol, pe of products = 250 kj mol. 7. is the reaction in # 6 exothermic or endothermic? explain. exothermic. the Δh is 100 kj mol which means heat is released. 8. how could you lower the activation energy for the reaction in #6? add a catalyst. A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants and ΔH is ... Summary of Exothermic Reactions: • More energy is by the reactants than is needed by the products • The excess energy is given off as • Heat input is often needed to provide activation energy to start the reaction • Heat from the reaction then keeps the reaction going Potential Energy Diagram Worksheet ANSWERS 1. Labeled Endothermic And Exothermic Graphs are a theme that is being searched for and liked by netizens today. You can Save the Labeled Endothermic And Exothermic Graphs here. Get all royalty-free pic. We Have got 13 images about Labeled Endothermic And Exothermic Graphs images, photos, pictures, backgrounds, and more.
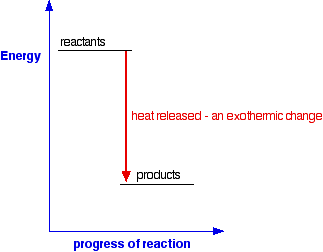
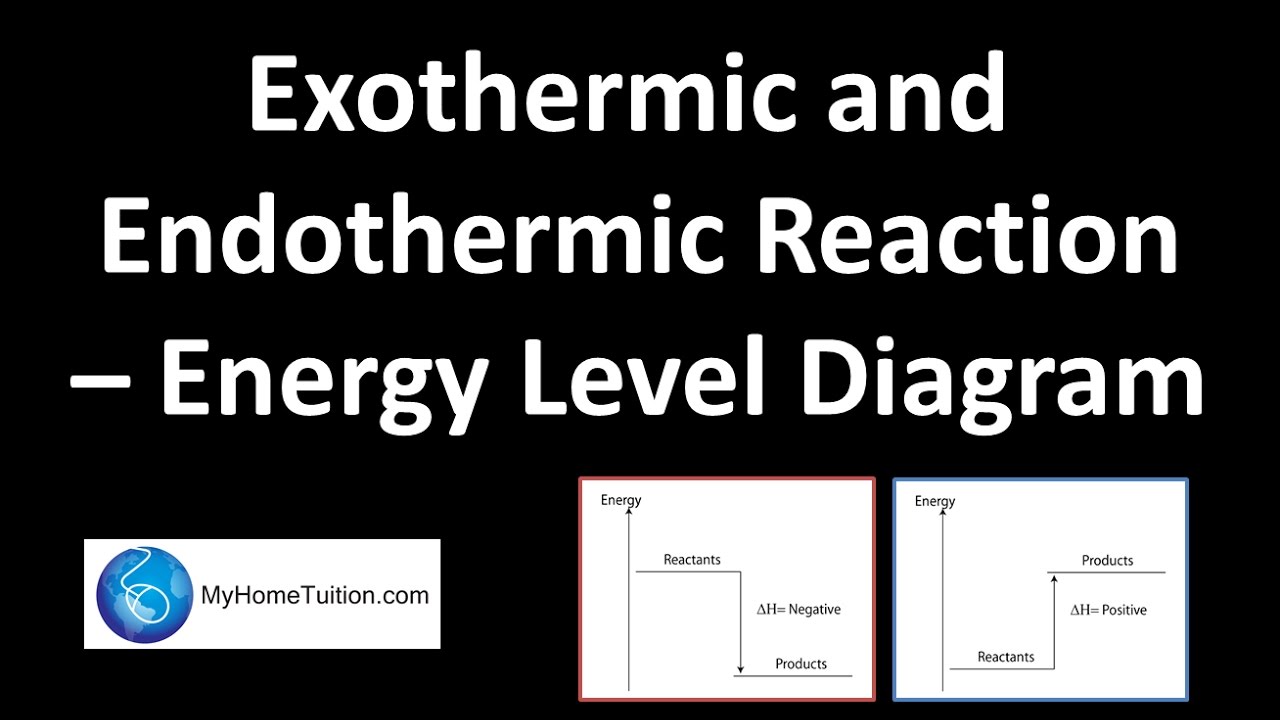
To correct for this, we introduce the concept of , which is much more commonly used by chemists.The enthalpy of reaction is defined as the internal energy of the reaction system, the product of pressure and volume. It is given by: By adding the term, it becomes possible to measure a change in energy within a chemical system... However, sweating is not an exothermic reaction. Chemical reactions can involve the breaking of existing chemical bonds, the formation of new bonds, or both. The... Sublimation of solid CO The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The is the energy that must be provided to... Department of Energy and its contractors, in paper, from: U.S. Department of Energy Office of Scientific and Technical Information P.O. Box 62 Oak Ridge, TN 37831-0062 OSTI http://www.osti.gov Phone: 865.576.8401 Fax: 865.576.5728 Email: *******@****.*** Available for sale to the public, in paper, from: U.S. Department of... Exothermic Reaction Potential Energy Diagram. Here are a number of highest rated Exothermic Reaction Potential Energy Diagram pictures upon internet. We identified it from honorable source. Its submitted by executive in the best field.
In order to effectively initiate a reaction, (kinetic energy) to bring about this bond disruption. More about this further on. And there is often one additional... Each atom-to-atom bond can be described by a potential energy diagram that shows how its energy changes with its length. When the bond absorbs energy (either from... The change in Gibbs free energy during a reaction provides useful information about the reaction's energetics and spontaneity (whether it can happen without added energy). We can write out a simple definition of the change in Gibbs free energy as:Δ, G, equals, G, start subscript, start text, f, end text, i, n, a, l, end... Or answer a question place a check mark in the box D next to that step. Does this potential energy diagram represent an exothermic or an endothermic reaction. Chemical potential energy is an objects potential to undergo an exothermic reaction. TRUE - Work is a form of energy and in fact it has units of energy. " Now, Sam and Julie are curious about the difference between an endothermic and an exothermic reaction. Consider the reaction mixture—salt plus water—as the... Depiction of an energy diagram In a chemical reaction, some bonds are broken and some bonds are formed. During the course of the reaction, there exists an... proof Endothermic vs. exothermic reactions This is the... potential energy and progress of a reaction over time....
During a chemical reaction, energy is either released or _____. absorbed. true or false: Physical and chemical changes can be either exothermic or endothermic changes. true. true or false: In exothermic reactions, the energy required to break the bonds in the reactants is greater than the energy released as the products form.
Of the collisions shown in the diagram, only collision 1 may possibly lead on to a reaction. If you haven't read... For a simple over-all exothermic reaction, the energy profile looks like this: Note: The only difference if the reaction was endothermic would be the relative positions of the reactants and products lines. For an...
In exothermic reactions H 2 is lower than H 1. Examples: Manufacture of ammonia in the Haber process; i.e. N 2(g) + 3H 2(g) → 2NH 3(g); ΔH=-46Kjmol-1; Dissolving sodium hydroxide pellets in water, The temperature of the resulting NaOH (aq) is higher than the temperature of water at room temperature.; This implies the internal temperature of products is lower than the reactants' original ...
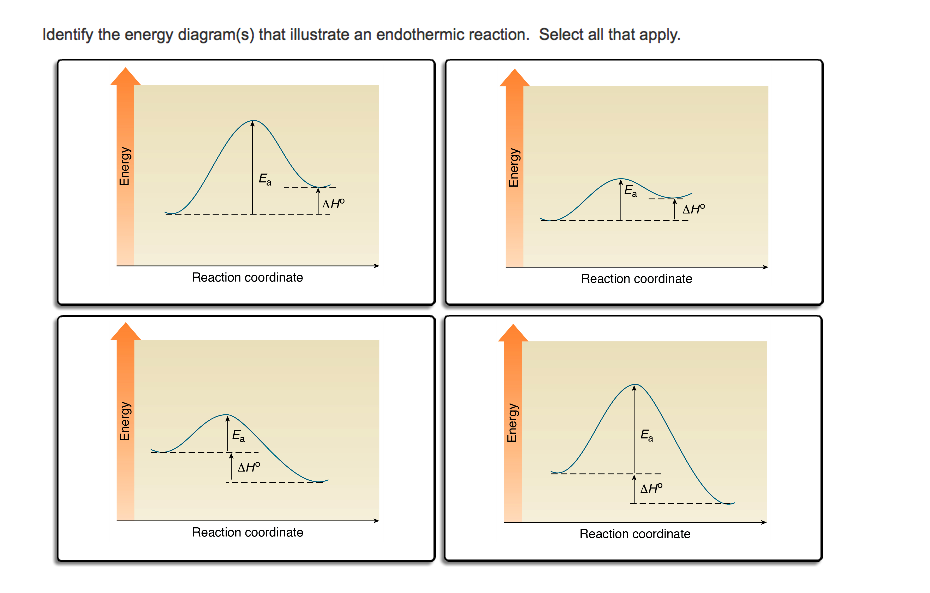
Is the overall reaction as shown exothermic or endothermic. Which of the letters a f in the diagram represents the potential energy of the products. A potential energy diagram shows the change in energy during a reaction. Label the axis pe of reactants 350 kj mol ea 100 kj mol pe of products 250 kj mol. Potential energy diagram worksheet answers 1.
and solve the equation to find the enthalpy change of reaction. ΔH + 2(805) + 436 = 1077 + 2(464) ΔH = 1077 + 2(464) - 2(805) - 436 ΔH = -41 kJ mol -1 Breaking bonds is endothermic; making bonds is exothermic There... Let's look at the hydrogen case on an energy diagram. Putting in +436 kJ per mole of hydrogen molecules (the...
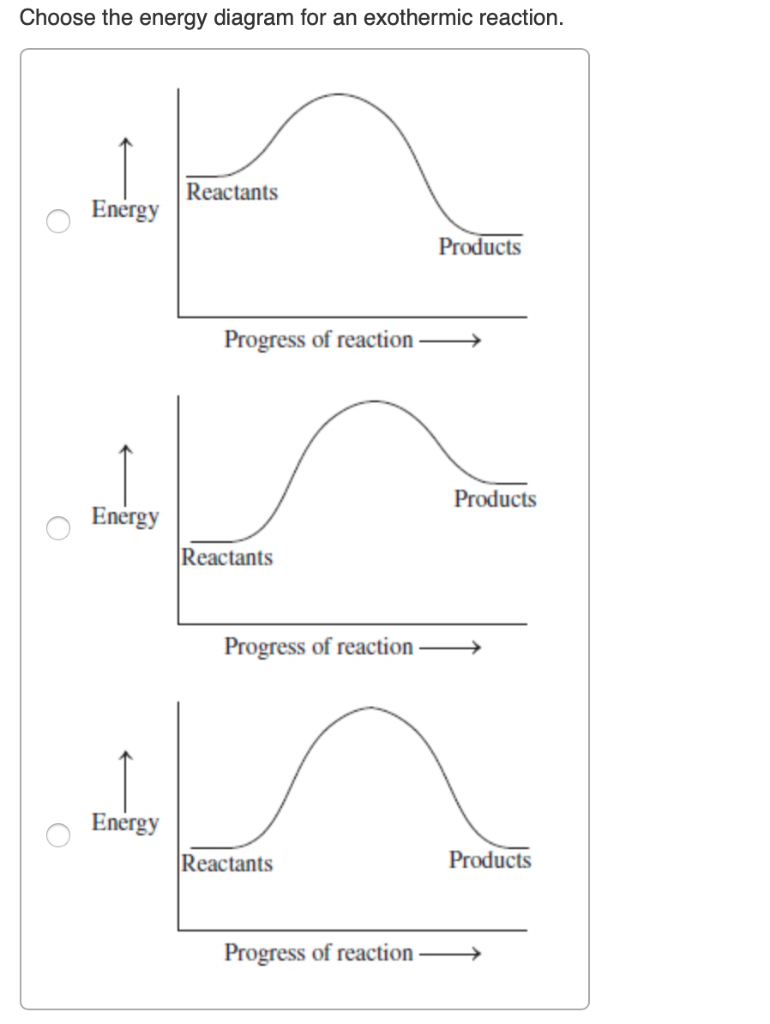
Here are a number of highest rated Energy Profile Diagram Exothermic pictures on internet. We identified it from well-behaved source. Its submitted by government in the best field. We take this kind of Energy Profile Diagram Exothermic graphic could possibly be the most trending topic similar to we allocation it in google lead or facebook.
A. energy transfer from a reaction system to its surroundings B. energy transfer as electromagnetic waves C. energy transfer by currents of moving liquid or gas D. energy transfer by direct contact 2. What is a chemical reaction system? A. the reactants and products in a reaction B. a diagram used to show energy changes in a reaction
Thebetween the and the is given the symbol ΔH (pronounced 'delta H'). This is the amount of that is given or taken during a
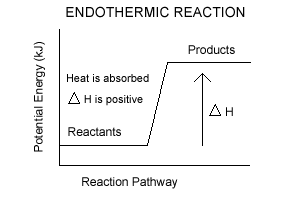
What are the two main sources of chemical energy? A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Potential energy diagrams for endothermic and exothermic reactions are described. Diagrams of activation energy and reaction progress are given.
Here are a number of highest rated Exothermic Reaction Energy Diagram pictures upon internet. We identified it from trustworthy source. Its submitted by management in the best field. We endure this kind of Exothermic Reaction Energy Diagram graphic could possibly be the most trending topic bearing in mind we allowance it in google plus or facebook.
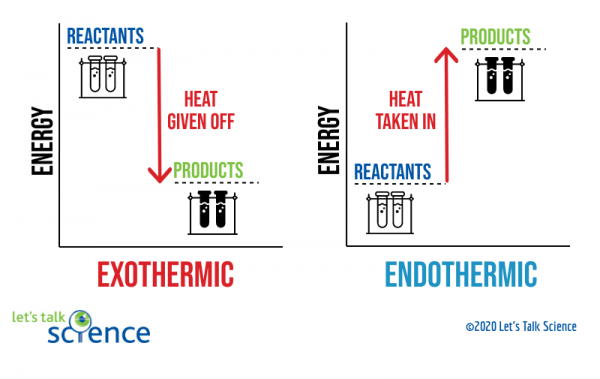
This reaction is "endothermic" since ΔH is positive. Since the value of ΔH is positive it also means that the products have more potential energy than the reactants. The difference in energy was absorbed from the surroundings. 4. Exothermic & Endothermic Reactions -Exothermic Reaction: Heat is released to the surroundings
Energy Level Diagram For Exothermic Reaction. Here are a number of highest rated Energy Level Diagram For Exothermic Reaction pictures upon internet. We identified it from trustworthy source. Its submitted by organization in the best field.
Energy is required to break bonds, and since the strengths of different kinds of bonds differ, there is often a significant overall energy change in the course of a reaction. In the combustion of methane, for example, all six bonds in the reactant molecules are broken, and six new bonds are formed in the product molecules...
The reaction is exothermic and so purple iodine vapour is formed, and probably dark grey solid iodine... Taking sodium chloride as an example: We need to supply the energy to break the attractions between the ions... The diagram shows that the overall change involving the halide ions is endothermic - the green arrow is pointing...
In this chemical reactions worksheet students use two graphs of chemical reactions to identify the activation energy heat content of the reactant and products and determine if the reaction is exothermic or endothermic. Is the above reaction endothermic or exothermic. Label the location of. Mar 23 2018 Potential Energy Diagram Worksheet ANSWERS 1.
Energy profile in an endothermic reaction. The energy profile in an endothermic reaction is the opposite of exothermic, and the reactants are at lower energy than products. The difference between them is the energy supply or heat absorbed. Spontaneity. The spontaneity of the reaction is measured by the change in enthalpy and entropy.
Explore the 4 factors affecting rates of a chemical reaction and how they affect it. 9. Draw the energy diagram of an exothermic reaction showing: energy of reactants & products, uncatalyzed activation energy, catalyzed activation energy, transition state (activated complex), and energy of the reaction (heat of reaction) 10.
the reaction written above exothermic or endothermic? Explain. The enthalpy change deals with breaking two mole of O-H bonds and the formation of 1 mole of O-O bonds and two moles of H-H bonds ( ). The sum of the... 7 kJ/mol which is an output (released) energy = 872.8 kJ/mol + 498.7 kJ/mol = 1371.5 kJ/mol. Total energy...
Chemical Reactions Unit Miss E. Mac's Classic; Chemical Reactions Unit Miss E. Mac's Class Of; Chem I Unit 5 Reactions Chemical Reaction: a process in which one or more substances are converted into new substances with different physical and chemical properties Reactant: a substance that enters into a chemical reaction Product: A substance produces by a chemical reaction.


















0 Response to "40 energy diagram endothermic and exothermic reaction"
Post a Comment