40 lewis dot diagram for gold
As an example, a neutral atom of gold (Au) contains 79 protons in its nucleus and 79 electrons. The first principal energy level, which is the one closest to ... Cl2 Lewis Structure . Lewis Structure is a simple depiction of valence shell electrons in a molecule. It tells us how electrons are organized around specific atoms in a molecule. It is also known as electron dot structures because electrons are represented by dots in this representation.
Lewis Dot Structures Objectives: 1. Draw Lewis structures for atoms, ions and simple molecules. 2. Use Lewis structures as a guide to construct three-dimensional models of small molecules. 3. Determine the electron and molecular geometry of the produced molecules. Background: Scientists often create models to represent either a physical or ...
Lewis dot diagram for gold
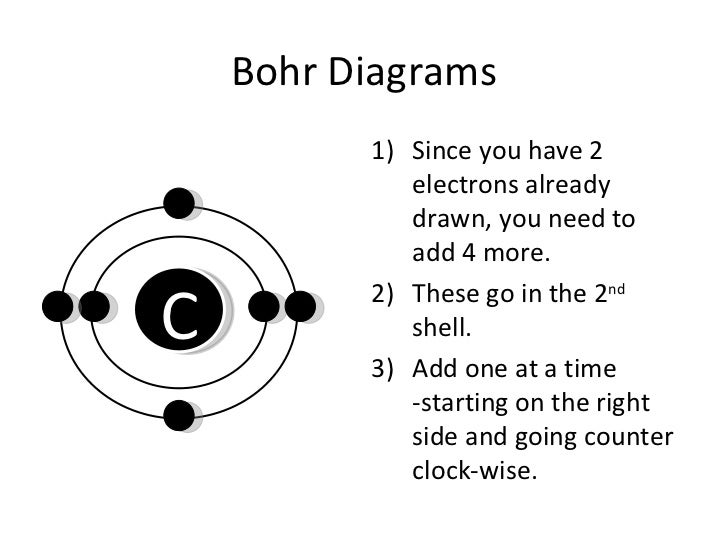
Is it possible to do a lewis dot structure for a metal such as gold or aluminum? For example, would "Al Al" be correct since aluminum is content with 6 valence electrons instead of the usual 8 (octet rule). How would you do gold or copper? My son's teacher has asked him to draw a lewis dot structure for a metal. Thanks! Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 4 dots around the symbol Lithium 3 7 3 3 Li Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
Lewis dot diagram for gold. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. Answer (1 of 6): Lewis dot formula had been invented to represent the number of electrons in the outermost shell (Valence shell) for the main group elements, to figure out the number of electrons which are needed to be lost or shared or gained by the element to complete its octet and to be used i... 6. Gold (Z=79) 7. Yttrium (Z=39) 8. Mercury (Z=80) 9. Xenon (Z=54) 10. Dysprosium (Z=66) For the following configurations determine the element, write the Lewis dot diagrams and write the orbital notation for the highest energy level. 1. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d5 2. 1s2 2s2 2p2 3. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 ... Finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Those are lewis dot structures. Let's learn how...
Jan 27, 2021 — Know and study the Gold valence electrons here for the thorough knowledge of this element. We are further going to provide every possible ... A step-by-step explanation of how to draw the MgO Lewis Dot Structure.For MgO we have an ionic compound and we need to take that into account when we draw th... Bohr Diagrams and Lewis Dot Structures What you've already learned in class and from readings You learned that Electrons can exist in different energy levels You learned that the # of Electrons in an atom are equal to the # of Protons in an atom You learned that the # of Valence Electrons are the outermost Electrons of an Atom What You're about to learn How to draw the Electrons around an ... This is the Bohr diagram for Osmium showing the electrons as they are in each shell of the element This is the Lewis Dot diagram for the element Osmium showing the number of electrons in the outer most shell of the element. History. Powered by Create your own unique website with customizable templates.
Aug 22, 2014 · The Lewis Dot Structure for a common Carbon atom is a C with one dot on all four sides: . . ... The Lewis Dot form of gold would be the Au (the symbol for gold) with a single dot. The dot ... What is the Lewis dot diagram for gold? What is the Lewis dot diagram for C2H2? What is the Lewis dot diagram for bromine? How is the Lewis dot diagram for neon determined? Magnesium has two electrons on its outer shell Each of the electrons will be shared with a Florine atom. This results in a compound # MgF_2#. Try it risk-free. Lewis Structures: Single, Double & Triple Bonds. from. Chapter 5 / Lesson 7. 69K. Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for ... The Lewis electron-dot diagrams for three substances are shown below. Describe, in terms of valence electrons, how the chemical bonds form in the substance represented in diagram 1. 23.Explain, in terms of element classification, why is an ionic compound. 24.Identify the type of bonding in solid potassium.
Lewis Structure of Al2O3. The concept of Lewis structure was first introduced by Gilbert N. Lewis in 1916. It is also known as the Lewis dot diagram or electron dot structure. It is the structural illustration of the position of the valence electrons, involved in the formation of a chemical bond, around the atoms inside a molecule.
Structure, properties, spectra, suppliers and links for: Gold(III) chloride, 13453-07-1, AuCl3.
The Lewis dot diagram for the covalent bonding of chlorine, ( Cl2 ), would be: When atoms are bonded ionically, the bond is represented by two dots between the element's chemical symbols. Ionic bonds are formed between charged particles (ions), so an example of an ionic compound would be NaCl, whose Lewis structure is: YouTube. chemistNATE.
A step-by-step explanation of how to draw the Br2O Lewis Dot Structure.For the Br2O structure use the periodic table to find the total number of valence elec...
Bohr Model Lewis dot diagram. 1. Determine the element's symbol 2. Determine the number of electrons 3. Determine number of valence electrons 4. Now draw your valence electrons ... Gold 196.967 111 [272] 11B Zinc 65.39 Cadmium 112.411 Hg Mercury 200.59 112 Cn [277] IllA Boron 10.811 Aluminum 26.982 Gallium 69.732 Indium 114.818 Thallium
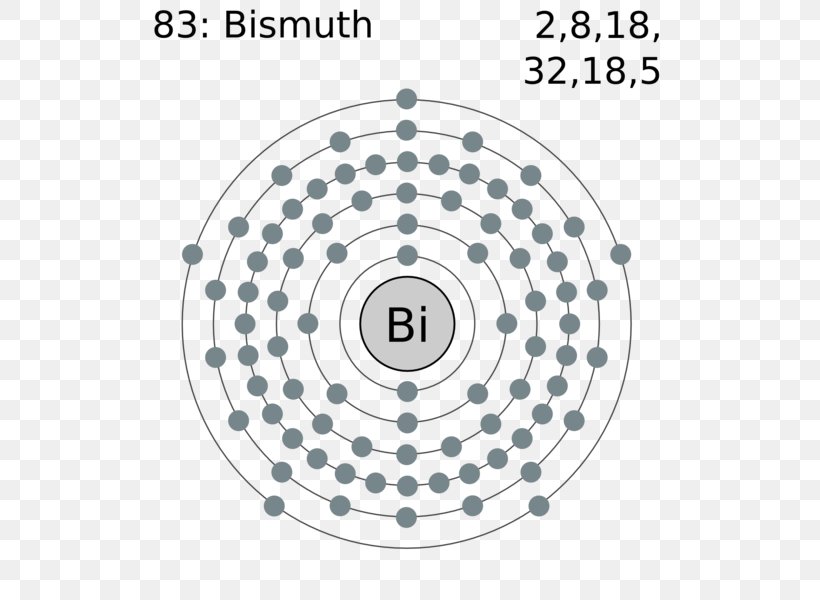
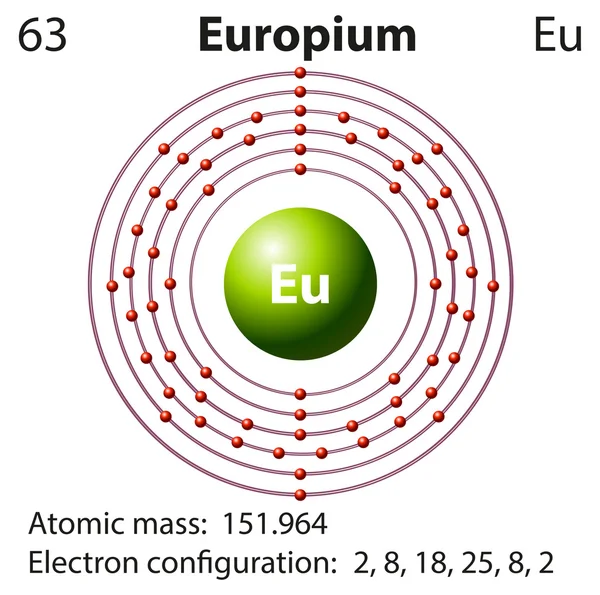
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
"Au" cdot Gold/Au (atomic number 79) has only one electron in its outer valence shell. The 10 xx 5d electrons in gold are in a filled energy level, leaving only one electron in the outer shell. The ground state configuration of Gold is [Xe] 5d^10 6s^1 The energy level difference between the 6s and 5d is small. This makes it possible for one of the two 6s electrons to rather be in the 5d orbitals.
Draw the Lewis Dot Structure. Notes: Scientists use. Lewis Dot Structures. to show the valance electrons of an element as dots. Since bonding involves the valance shell electrons only, it is only necessary to illustrate those outer electrons.
Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 4 dots around the symbol Lithium 3 7 3 3 Li
Is it possible to do a lewis dot structure for a metal such as gold or aluminum? For example, would "Al Al" be correct since aluminum is content with 6 valence electrons instead of the usual 8 (octet rule). How would you do gold or copper? My son's teacher has asked him to draw a lewis dot structure for a metal. Thanks!


![[DIAGRAM] Electron Dot Diagram Gold FULL Version HD ...](https://showme0-9071.kxcdn.com/files/406/pictures/thumbs/422761/last_thumb1351009921.jpg)






![[DIAGRAM] Electron Dot Diagram Gold FULL Version HD ...](https://www.vippng.com/png/detail/514-5142248_us-electron-dot-diagram-for-electron-dot-structure.png)


![[DIAGRAM] Electron Dot Diagram Gold FULL Version HD ...](https://i.stack.imgur.com/9CFV0.jpg)






/Silver-58b601c15f9b5860464c0e8f.jpg)




0 Response to "40 lewis dot diagram for gold"
Post a Comment