45 ch4 molecular orbital diagram
The energy level diagram of molecular orbitals of $\ce{CH4}$ is not clear to me. molecules molecular-orbital-theory. Share. Improve this question. Follow edited Apr 19 '16 at 21:25. Rajnish kumar. asked Apr 19 '16 at 20:30. Rajnish kumar Rajnish kumar. 23 4 4 bronze badges $\endgroup$ 1. 2 A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding.
A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.

Ch4 molecular orbital diagram
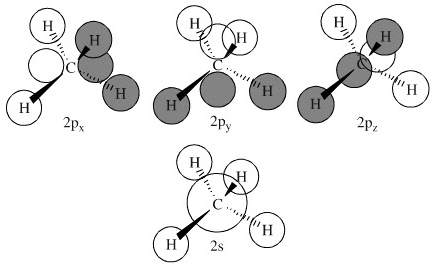
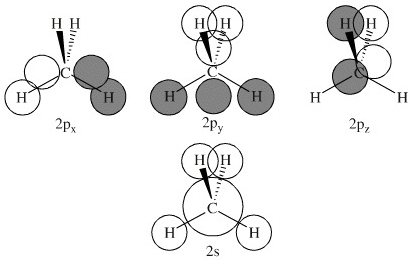
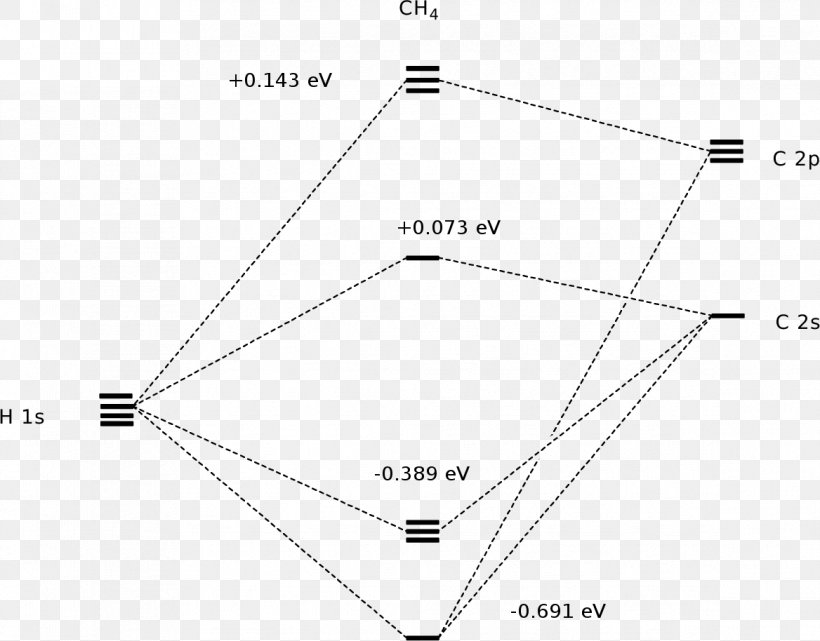
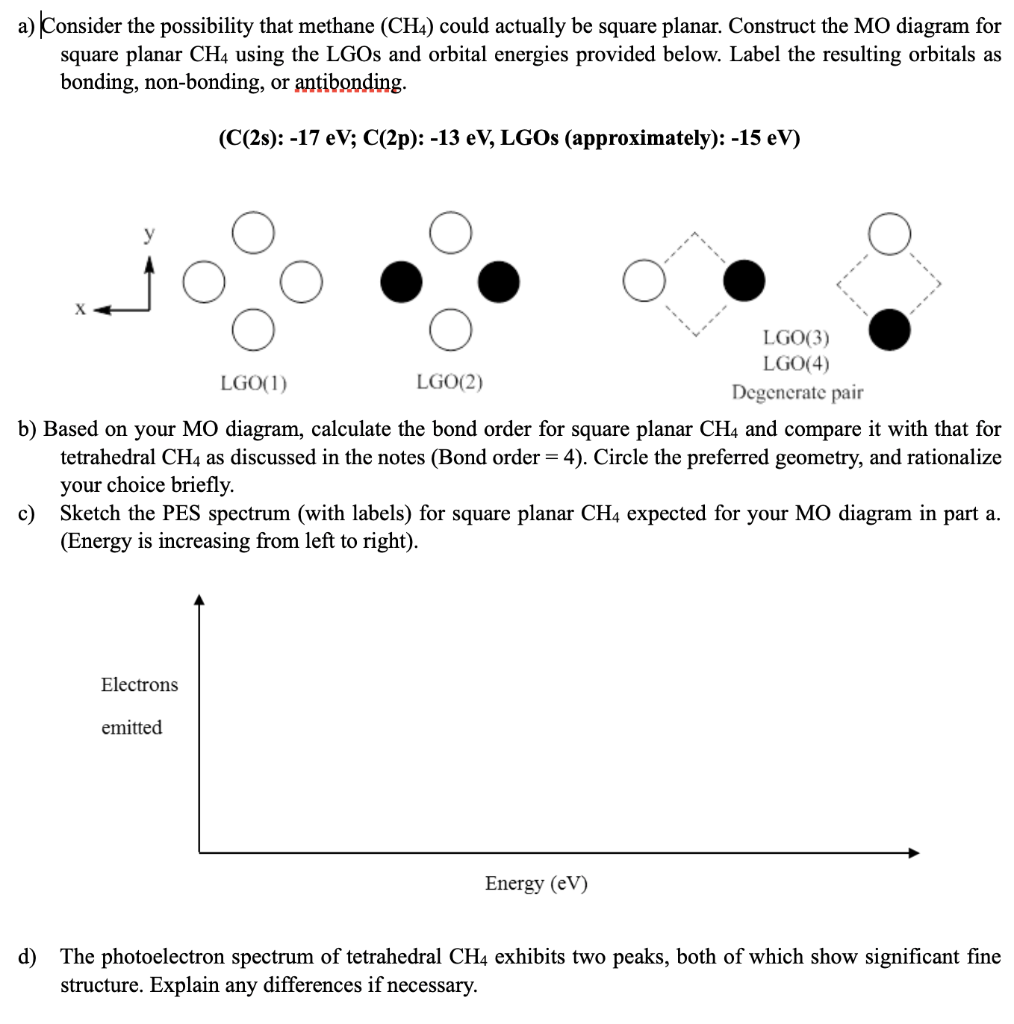
Generate the Molecular Orbitals for CH4 (Td), CH4 (D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us. Here is an energy level diagram showing how the 4 hydrogen 1s orbitals.Jan 18, · Using LCAO to Construct MOs for ... In methane, the four hybrid orbitals are located in such a manner so as to decrease the force of repulsion between them. Nonetheless, the four orbitals do repel each other and get placed at the corners of a tetrahedron. CH 4 has a tetrahedral shape. The sp 3 hybrid orbitals have a bond angle of 109.5 o. UCI Chem 131A Quantum Principles (Winter 2014)Lec 27. Quantum Principles -- CH4 Molecular Orbitals and Delocalized Bonding --View the complete course: http:...
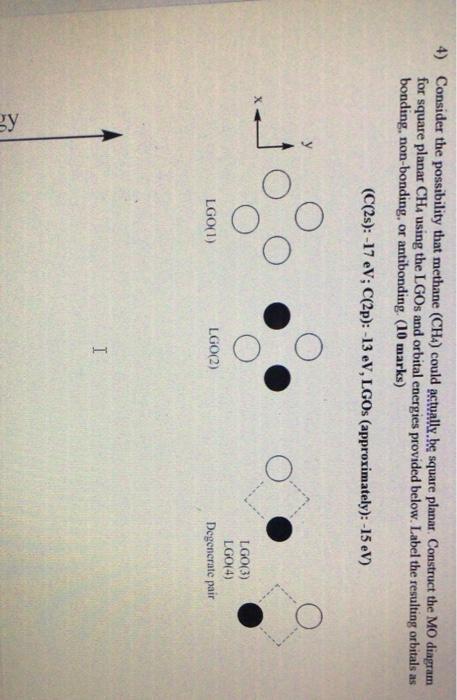
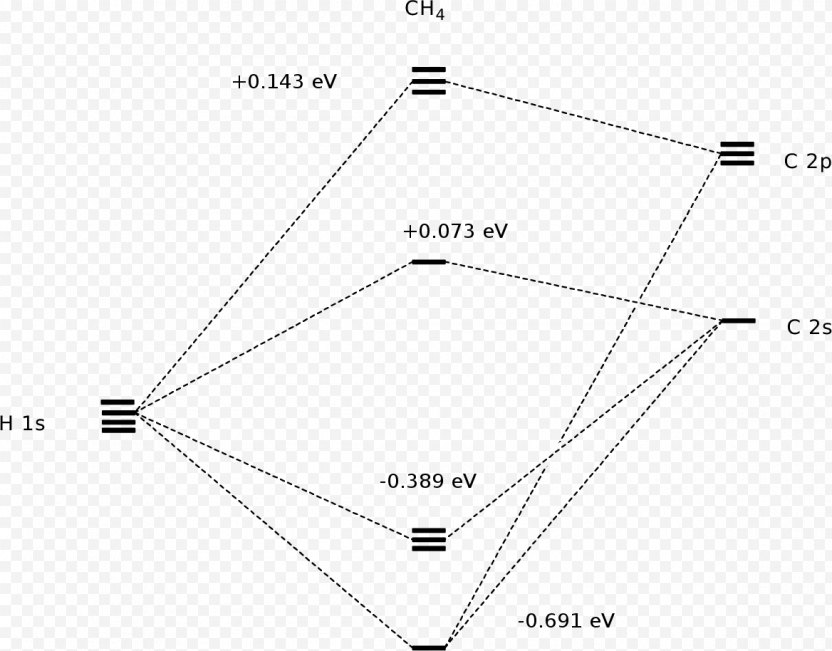
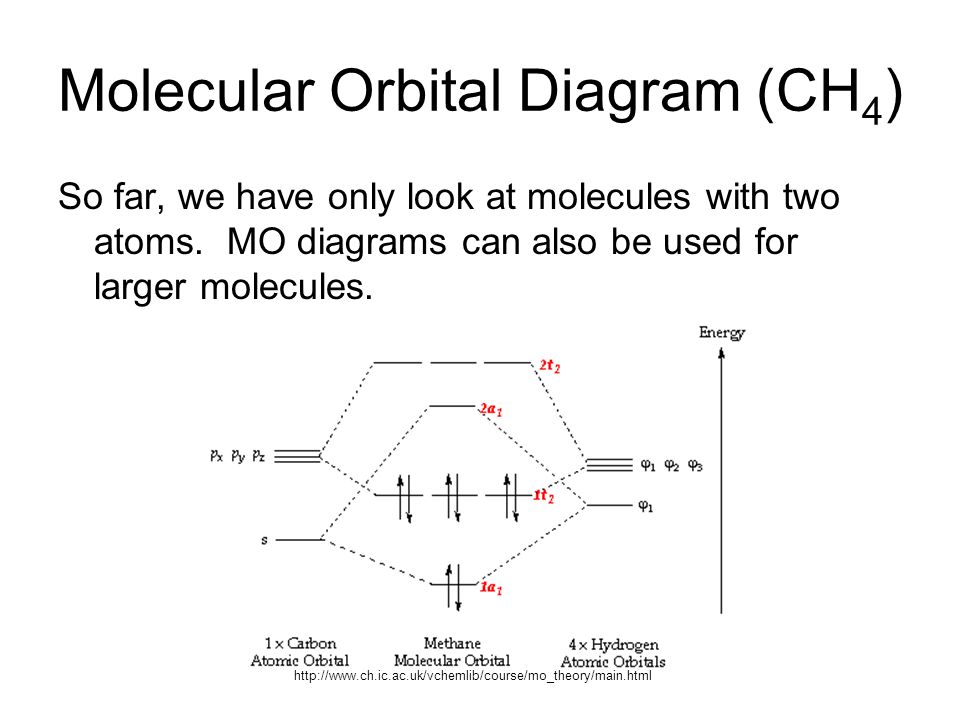
Ch4 molecular orbital diagram. 2 days ago · Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen. A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. 388) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two Construct a molecular orbital diagram for CH4 from a linear combination of symmetry adapted orbitals (group orbitals of the H atoms overlapping with the atomic orbitals of the Catom). Orient the molecule as shown in the picture on the left below. Assign the axes as shown in the picture on the right below. Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a...
The molecular orbital description of bonding in methane does several things for us. It should reconcile our valence-bond idea of electrons localized between carbon and hydrogen with the "delocalized" picture typical of the MO approach. It should tell us (quantitatively) about the energies of different electrons. For the molecule methane (CH4):a) Write the condensed electron configuration.b) Draw the filled atomic orbitals of the central atom.c) What should the bond a... Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals. 1. Begin with the Lewis Molecular Orbital of Methane, CH4. 1. The Lewis. Molecular Orbital theory (MO) is the most important quantum mechanical theory This particular diagram shows the orbitals for both the hydrogen atom and the. Ch.4 Molecular Structure and Orbitals STUDY Flashcards Learn Write Spell Test PLAY Match Gravity VSEPR model Click card to see definition 👆 structure around a given atom is determined principally by minimizing e- pair repulsion Click again to see term 👆 1/24 Previous ← Next → Flip Space Created by Jose_Lopez762
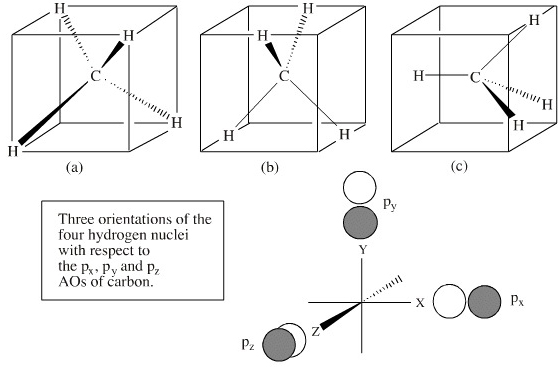
Methane (CH4) has tetrahedral geometry and Td point group symmetry. Derive a molecular orbital diagram for methane by performing the following steps: a. Determine the reducible representation (Γ) describing the symmetry of the four H 1s orbitals that are involved in σ bonding with the valence atomic orbitals of C. b. Express the Molecular Orbital Diagram for Methane. Sigma and pi covalent bond models have proven to be valuable tools for describing the structure and reactivity of simple molecules, such as methane and ethene. However, such models do not accurately represent the electron distribution within the molecules. In the case of methane, this model implies four ... Polyatomic Species: Molecular Orbitals. Polyatomic species like methane, CH 4, can be described in terms of molecular orbital theory, however, the diagrams can be very difficult to visualise. However, structures built up from hybrid atomic orbitals are much easier comprehend. UCI Chem 131A Quantum Principles (Winter 2014)Lec 27. Quantum Principles -- CH4 Molecular Orbitals and Delocalized Bonding --View the complete course: http:...
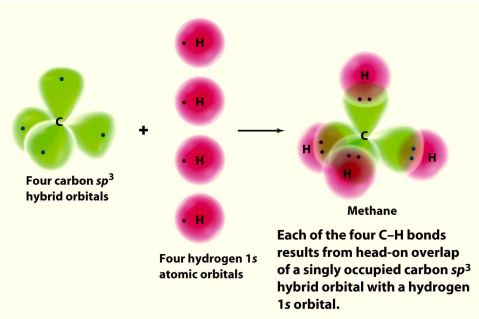
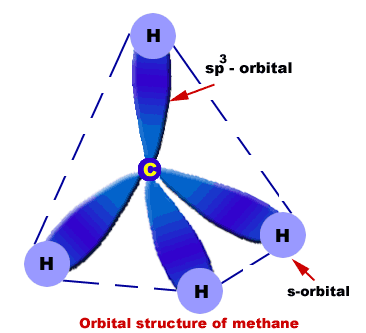
In methane, the four hybrid orbitals are located in such a manner so as to decrease the force of repulsion between them. Nonetheless, the four orbitals do repel each other and get placed at the corners of a tetrahedron. CH 4 has a tetrahedral shape. The sp 3 hybrid orbitals have a bond angle of 109.5 o.
Generate the Molecular Orbitals for CH4 (Td), CH4 (D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us. Here is an energy level diagram showing how the 4 hydrogen 1s orbitals.Jan 18, · Using LCAO to Construct MOs for ...



















0 Response to "45 ch4 molecular orbital diagram"
Post a Comment