40 ammonia electron dot diagram
...below, draw electron-dot diagrams for the following molecules: hydrogen (H2), ammonia (NH3) The steps involved in the electron-dot structure of given molecule are : Step 1 : First we have to All the electron-dot diagram of given molecule is shown below. webew7 and 17 more users found this... Valence electrons can be counted using a Lewis electron dot diagram. In carbon dioxide, for example, each oxygen shares four electrons with the central carbon. These four electrons are counted in both the carbon octet and the oxygen octet because they are shared. Carbon dioxide A Lewis dot diagram for carbon dioxide. Hydrogen and Lithium
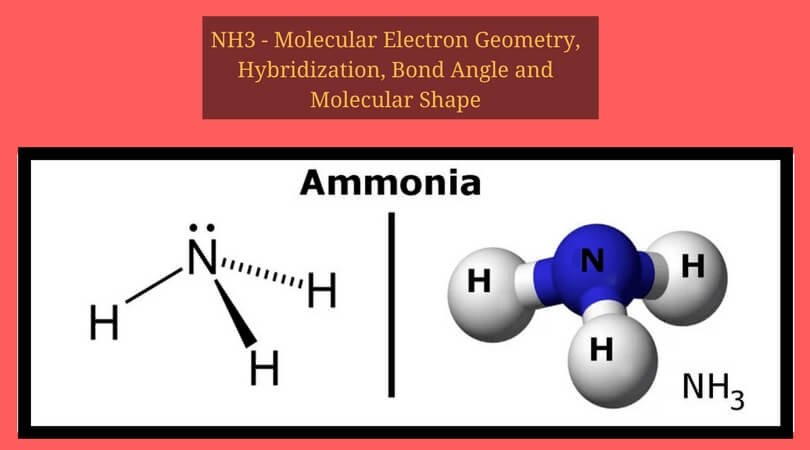
Ammonia (NH3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a ...Oct 25, 2016 · Uploaded by Wayne Breslyn
Ammonia electron dot diagram
Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton. In this blog post, we will learn about the Lewis dot structure, electron geometry... is represented by the dots in its Lewis electron-dot diagram? (1) the electrons in the fi rst shell (2) the electrons in the fourth shell (3) the protons in the nucleus (4) the neutrons in the nucleus 10 Based on Table S, an atom of which element has the strongest attraction for electrons in a chemical bond? (1) aluminum (3) magnesium Electron Dot Structure Of Cl2, H2O &Amp; Nh3 Class-X. Nh3Ammonia Dot Structure Class10Lesson4Carbon And Its Compoundscbse. 07:02. 71 2.2K. Lewis Diagrams Made Easy: How To Draw Lewis Dot Structures. 13:27. 20K 573.2K.
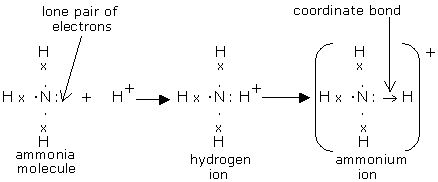
Ammonia electron dot diagram. Electron dot structure. (a) Electrovalent bonding: • Electron dot structure of Electrovalent compounds NaCl, MgCl. 2, CaO. • Characteristic properties of electrovalent compounds state of – existence, melting and boiling points, conductivity (heat and electricity), dissociation in solution and in molten state to be linked with electrolysis. Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in 1916. These diagrams are used as a shorthand notation to show the number of valence electrons in an atom. More complicated versions can be used to show the bond between different atoms in a... By drawing an electron dot diagram show the formation of Ammonium Ion [Atomic No. : N = 7 and H = 1]. A step by step explanation of how to write the lewis dot structure for nh3 ammonia or nitrogen trihydride. You have to draw the lewis struc...
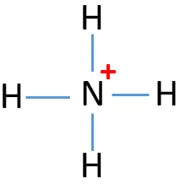
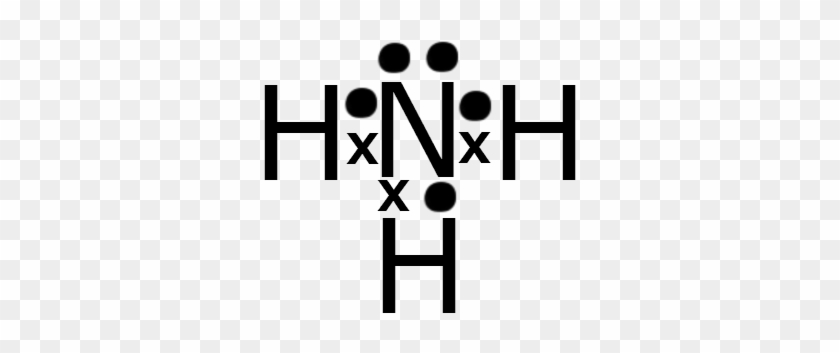
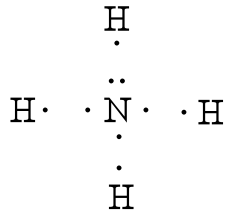
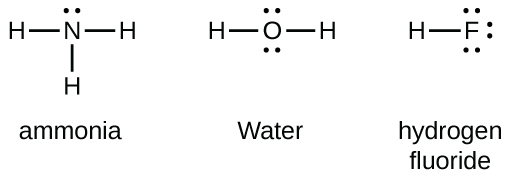
Ammonia NH3 Electron dot diagramN(eed) H(ave)N: 8 N: 53H: 2x3= 63H: 1 x 3 =3Nitrogen is an exception, a noble gas. It doesn't go with the octet rule. In order to be happy, it needs only2Hydrogen has 3 this time, so multiply.Add columns.8 + 6 = 145 + 3 = 8Now find the difference between the 2.14... -In ammonium ion, the lone pair on nitrogen atoms of ammonia has the ability to fully share its pair with hydrogen ions, thus forming a coordination bond with ...1 answer · Top answer: Hint: Ammonium ion consists of nitrogen and hydrogen atoms. It has one nitrogen atom and 4 hydrogen atoms.Complete step-by-step answer:-Ammonia consists ... This incomplete dot and cross diagram shows only the bonding pairs of electrons. Finally, add in the non-bonding outer electrons. Three of these are shared, which leaves two electrons that do not take part in bonding. The complete dot and cross diagram for ammonia. Electron Dot Diagrams. The electrons in an atom's outer energy level are the electrons that are These electrons can be represented in a diagram called an electron dot diagram. these structures can be referred to as valence structures. ammonia, NH3 1. Nitrogen atom has 5 valence electrons 2...
Describe the electron dot diagram for a sodium atom. ... (C3v symmetry): Valence Bond Approach: Draw a Lewis structure for ammonia and assign hybrid orbitals for the nitrogen atom. Draw an energy ... Electron configurations are expressed as diagrams or as a sequence of numbers. For example, sodium has an atomic number of 11, so it has 11 protons and 11 electrons. This is shown as an electron 'dot' diagram below, or can be expressed as 2, 8, 1 where the sequence of numbers... Ammonia (NH3) act as a lewis base because of presence of lone pair on the nitrogen atom. Replacement of one, two and three H's on the ntrogen with -CH3 generat … View the full answer. Transcribed image text : Assemble ammonia NH3. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element 's symbol. Electron dot diagrams would be the same for each element in the representative element groups.
For example, the electron dot diagram for iron (valence shell configuration 4s23d6) is as follows: Elements in the same column of the periodic table have Thus the electron dot diagrams for the first column of elements are as follows: Monatomic ions are atoms that have either lost (for cations) or...
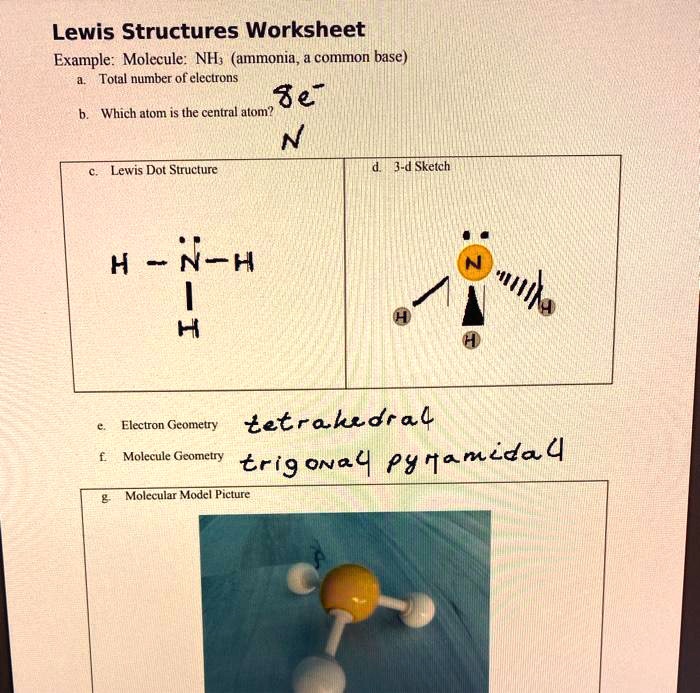
Electron Dot Diagrams / Lewis Structures - . atom and covalent compound diagramming. electron dot diagrams. shows. Electron-dot formula for Ammonia (NH3) H N H Here is a Nitrogen atom (5 val e-'s) and three Hydrogen atoms (1 val e- each) H.
Ammonia (NH3) is sp3 hybridized, or to be more specific, the central atom of ammonia, nitrogen. We'll look at how to figure out if NH3 is hybridized in this article. Lewis structures add lines between atoms to represent shared pairs in a chemical bond, extending the concept of the electron dot diagram.
Use the electron dot structure below. Circle each unshared pair of electrons in a water molecule. O H H 5. Complete the electron dot structure for each molecule. Each molecule contains only single covalent bonds. H H OO H C H HH H N H H a. NH 3 b. H 2 O 2 c. CH 4 6. A chemical bond formed when atoms share two pairs of electrons is called a(n ...
Lewis structure, electron dot diagram, electron dot structure... What information can you get from an electron dot diagram? The electron dot diagram is a way of working out the bonding of a molecule.
Molecular formula of ammonia is NH4. Draw eletron-dot and line structures for ammonia molecule.
Ammonia is a compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct characteristic of...
Chemistry 11: Electron Dot Diagrams. 4 hours ago 2. Electrons in the Lewis Structure (electron dot diagram) are paired to show the bonding pair 8 hours ago This photo about: Electron Dot Diagram for Ammonia, entitled as Chem Supplemental Instruction Dean Students Fice Nickel Lewis Dot...
Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked examples for chemistry students. In the Valence Structure for ammonia, the bonding pairs of electrons, which may or may not be circled in the Lewis structure, are replaced by a...
May 17, 2021 · Red dot is FE estimated by three independent NMR tests. ... between each two successive steps in the free energy diagram. ... reduction of nitrate to ammonia via direct eight-electron transfer ...
Number of bonded pairs and lone pairs of electrons present in the central atom of ammonia molecule are · Draw an electron dot diagram to show the formation of ...1 answer · Top answer: Step 1: Find valence e - for all atoms. Add them together. N - 5 H - 1x3 = 3 Total = 8 Step2: Find octet e - for each atom and add them together.N - 8 H ...
Learn how to draw dot- and- cross diagrams for atoms, ions and molecules, showing all the electrons in each shell (principal quantum shell). You will need to know how to determine the electronic configuration, before using the method described below, to draw the dot- and- cross diagrams.
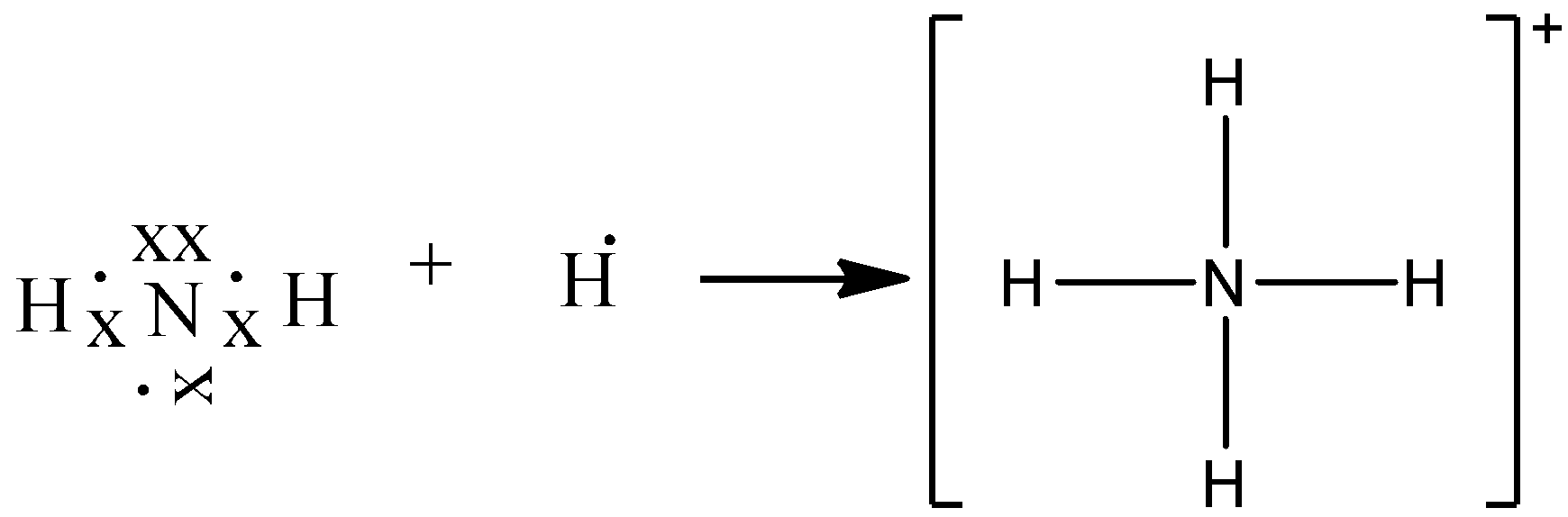
With the help of an electron dot diagram show the formation of hydronium ion and ammonium ion from a water molecule and an ammonia molecule respectively.
> Draw the electron dot stucture of ammonia molecule. > Assertion: Ammonia shows a trigonal pyramidal molecular structure. Reason: In the structure of ammonia, three atoms are attached to the central atom and thus, shows tetrahedral electron pair geometry.
To see all my Chemistry videos, check outhttp://socratic.org/chemistryThis is an introduction to the basics of VSEPR Theory. VSEPR theory is a set of rules f...
Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. And #3xxN-H# bonds, and one nitrogen-centred lone pair of electrons.
With this information draw a dot diagram for each element in the chart. Remember only the valence electrons are represented in the diagram not. Dot dot. Nnamdi Ammonia hi.
![Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1 ].](https://i.ytimg.com/vi/t_Z8qSTAfr0/mqdefault.jpg)
Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1 ].
5. Complete the electron dot diagrams below and fill in the rest of the table. (the first one has been done for you) Electron Dot Diagrams for (SiH4) 10. DOUBLE and TRIPLE BONDS: In your exercise book, draw electron dot diagrams for the following molecules. The first four have at least one double...
Find step-by-step Chemistry solutions and the answer to the textbook question A) Draw an electron dot diagram of ammonia.
This chapter will explore yet another shorthand method of representing the valence electrons. The method explored in this lesson will be a visual representation of the valence electrons. We will, as we observed in the previous lesson...
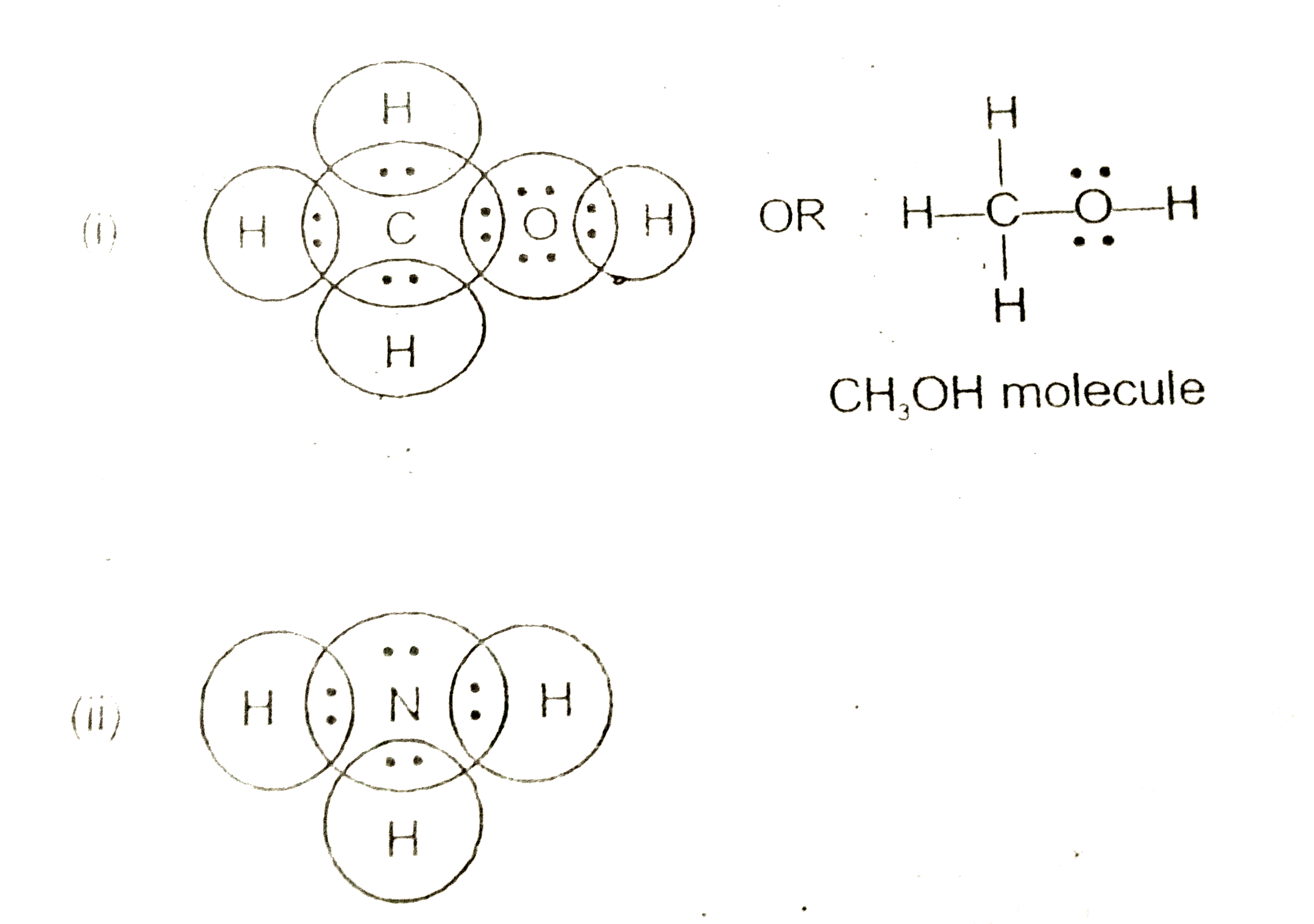
Electron dot structure valence electrons are represented by dots placed around the chemical symbol. Each hydrogen atom is covalently bonded to the nitrogen via an electron pair and another pair of electrons is attached to the nitrogen atoms outer shell.
Electron Dot Structure Of Cl2, H2O &Amp; Nh3 Class-X. Nh3Ammonia Dot Structure Class10Lesson4Carbon And Its Compoundscbse. 07:02. 71 2.2K. Lewis Diagrams Made Easy: How To Draw Lewis Dot Structures. 13:27. 20K 573.2K.
is represented by the dots in its Lewis electron-dot diagram? (1) the electrons in the fi rst shell (2) the electrons in the fourth shell (3) the protons in the nucleus (4) the neutrons in the nucleus 10 Based on Table S, an atom of which element has the strongest attraction for electrons in a chemical bond? (1) aluminum (3) magnesium
Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton. In this blog post, we will learn about the Lewis dot structure, electron geometry...




![Solved] Two students made the Lewis dot diagrams of NH3. The ...](https://us-static.z-dn.net/files/da4/66daa2f82875e459e2b8f9f507019d20.png)







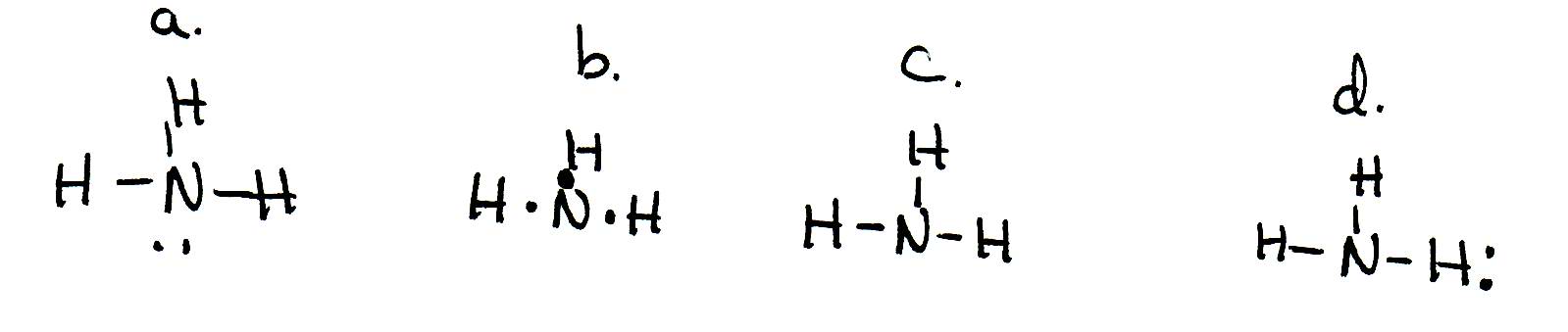







0 Response to "40 ammonia electron dot diagram"
Post a Comment