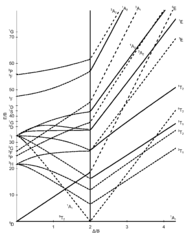
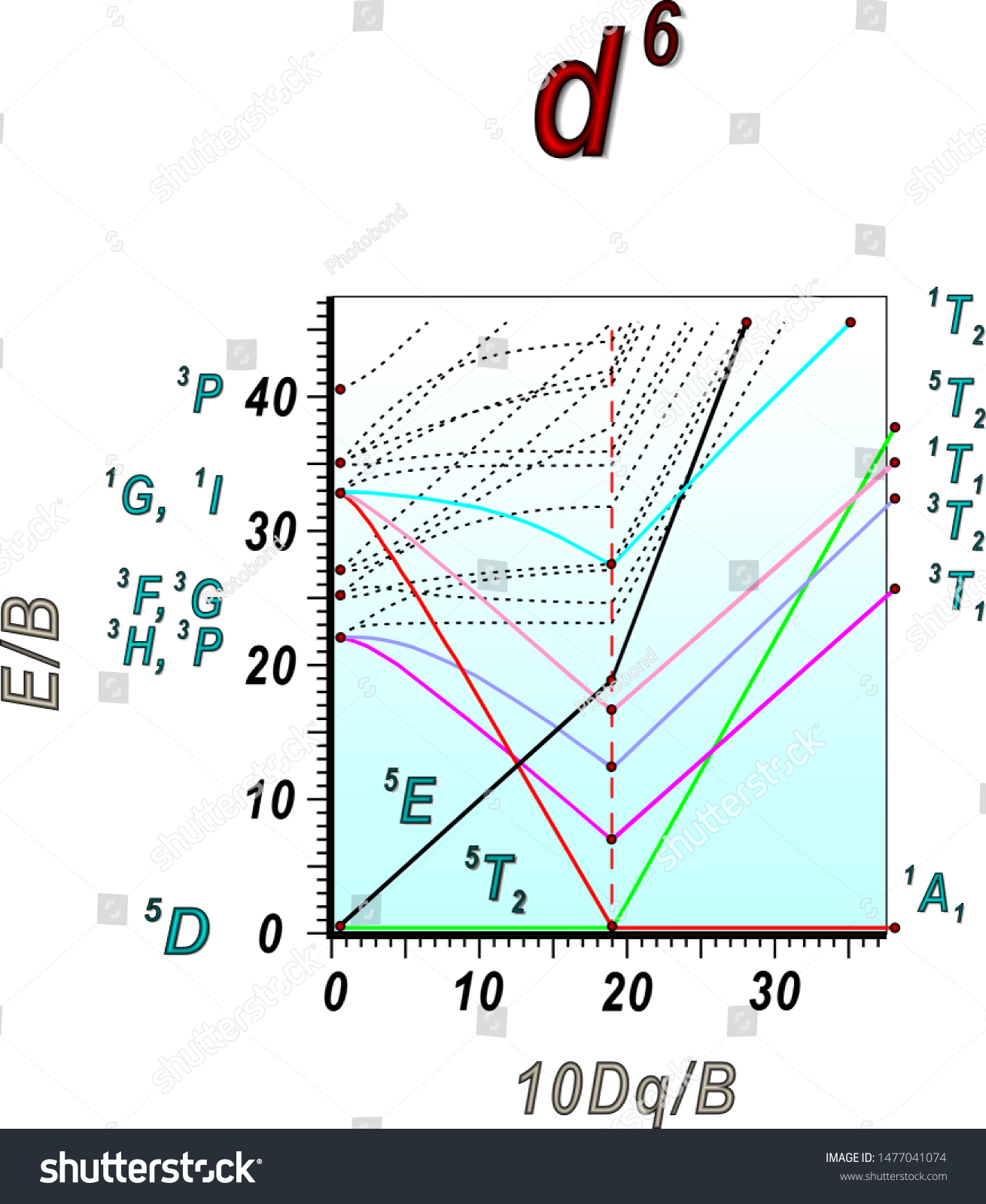
44 tanabe-sugano diagram
I am a graduate student in Chemistry in an Inorganic class. We have touched on [Tanabe-Sugano diagrams](http://chemwiki.ucdavis.edu/@api/deki/files/976/D5_Tanabe-Sugano_diagram.png) in class, but did not go into deep explanation. The homework problems I have been given use these diagrams, but I am not sure how to use them. What I understand: These diagrams show the allowed transitions for both high and low spin complexes. I thought that term symbols were used to read these diagrams, but the E,... Current PhD candidate and working towards some in-house teaching certifications. One project is developing your own syllabus for a course you plan to teach post-graduate school. As an organotransition metal chemist, I'd expect to be teaching an Undergraduate Inorganic Chemistry course at some point. Curious to know what concepts you wish were taught more/better in undergrad Inorganic Chemistry, as well as things you felt were not useful/not needed. For example my PI doesn't bother with Tanabe...
If you look in the Tanabe-Sugano diagram for iron (III), you'd expect a very weak absorption, given that there are no spin-allowed transitions from the ground state. But as we all know, an aqueous solution of ferric chloride has a really intense yellow color. How can that be explained?

Tanabe-sugano diagram
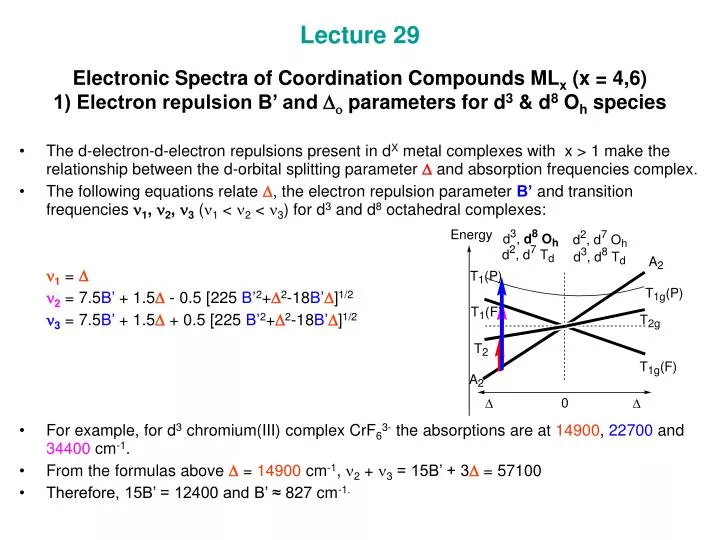
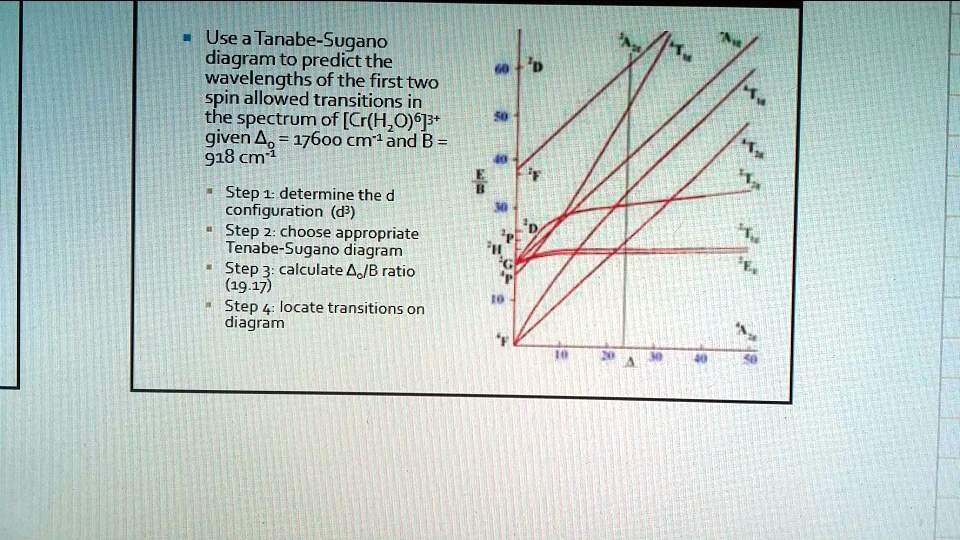
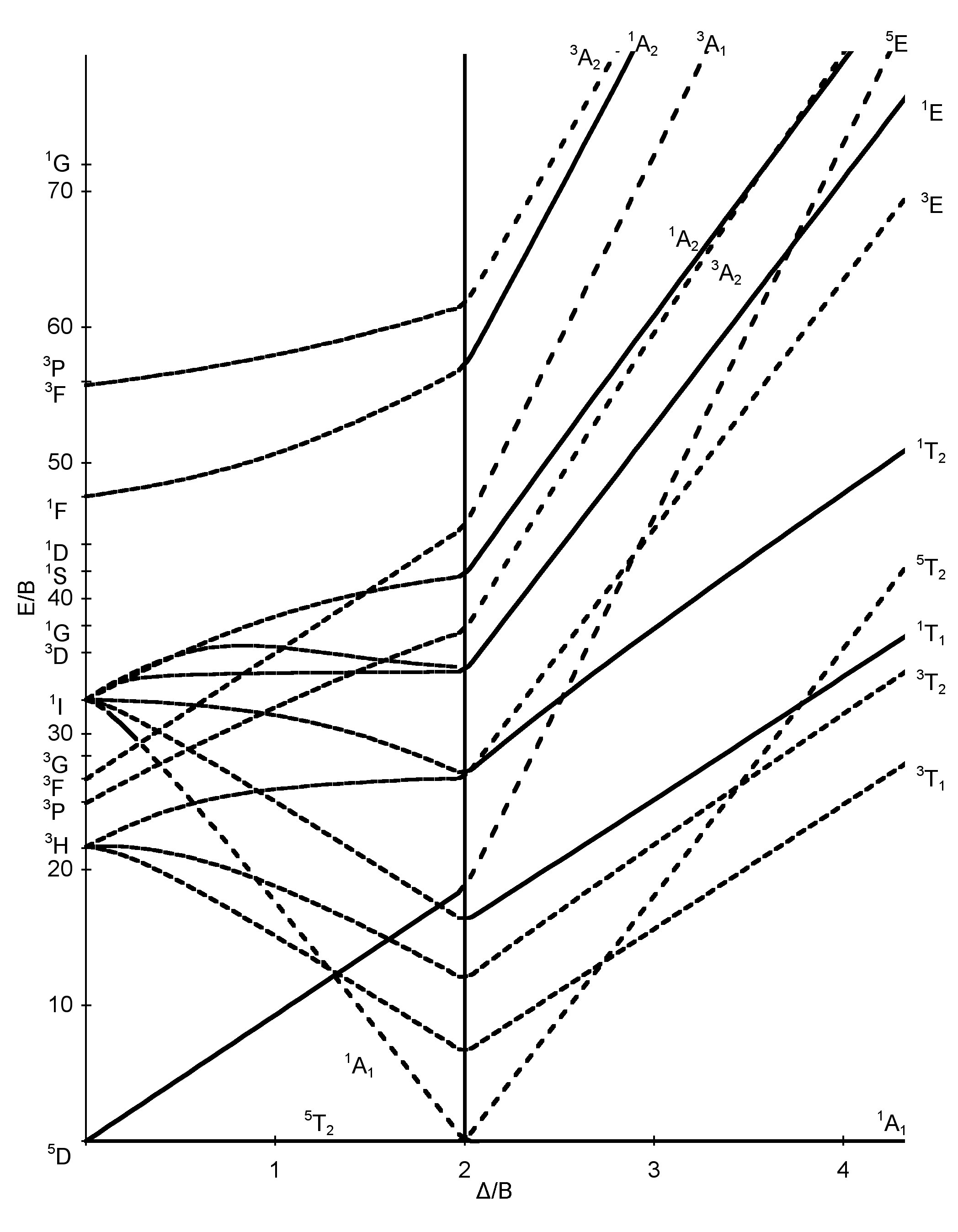
The spectrum of [Ni(Metu)6]2+ (Metu = N-methylythiourea) has peaks of moderate intensity at 8800, 13,100 and 19,800 cm-1. Estimate the value of Dq and B. **The following absorption bands are found in the spectrum of [Cr(CN)6]3-: 2640 angstrom, 3100 angstrom and 3780 angstrom. Calculate appropriate values of B' and Dq.** [Here's](https://upload.wikimedia.org/wikipedia/commons/thumb/c/c5/D3_Tanabe-Sugano_diagram.png/800px-D3_Tanabe-Sugano_diagram.png) the Tanabe-Sugano diagram for this system. The ground state for the d3 ion is 4A2g, so there are three spin-allowed transitions. However, I can't seem to find an x axis value where all the energy rat... I'm currently having trouble understanding Tanabe Sugano diagrams, especially when I'm asked to determine the transitions (T1g to T2g or something like that). Do you guys have any tips or things I should look out for when trying to determine these transitions?
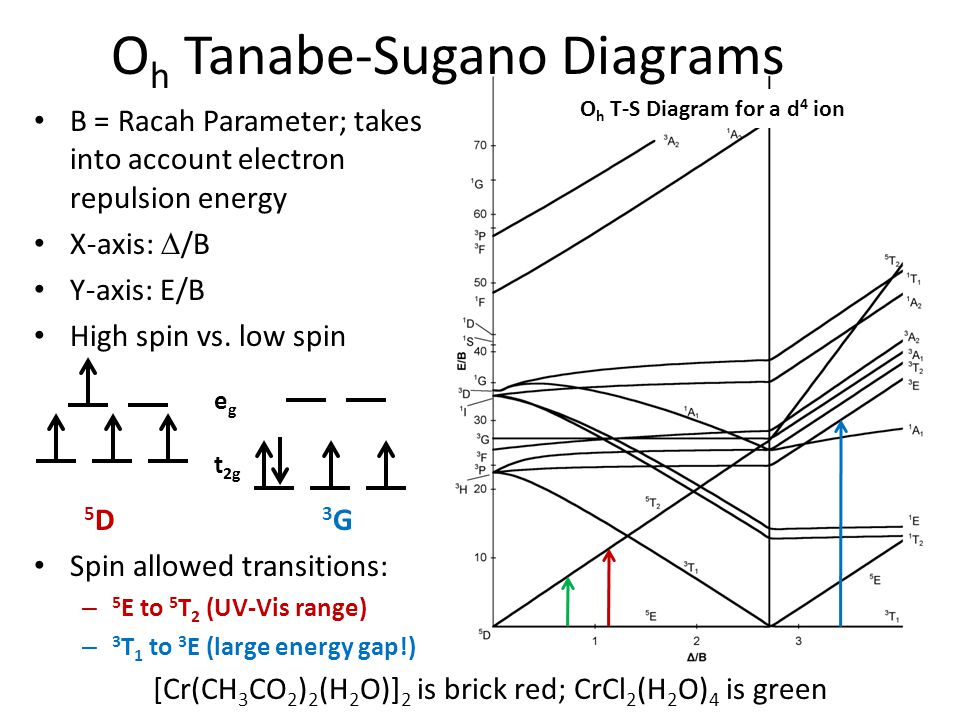
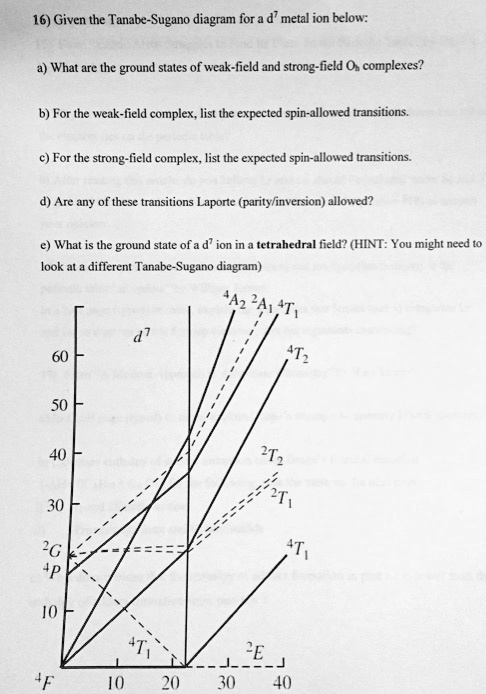
Tanabe-sugano diagram. I've been teaching my students about the Tanabe-Sugano diagram and what each individual term symbol represents in terms of the electronic structure for metal ions. For a d3 ion, there are three spin-allowed transitions from the 4A2g ground state - t2g^3 -: 4T2g <-- 4A2g 4T1g <-- 4A2g 4T1g (P) <-- 4A2g It's simple enough to deduce that the first two spin-allowed transitions corresponds to t2g(2) eg(1) <-- t2g(3), and that the last spin-allowed transition is t2g(1) eg(2)... I'm confused as to how one determines this value--I understand it's dependent on the chart, but how exactly can you find Dq/B? I am a graduate student in Chemistry in an Inorganic class. We have touched on Tanabe-Sugano diagrams in class, but did not go into deep explanation. The homework problems I have been given use these diagrams, but I am not sure how to use them. What I understand: These diagrams show the allowed transitions for both high and low spin complexes. I thought that term symbols were used to read these diagrams, but the E, T, and A designations look more like molecular orbital labels. Somehow, the de... I understand the importance of the diagrams for understanding and predicting d-d bands, but does anyone actually still use them? None of the lecturers in my department have ever actually used them. What about you guys, ever found any actual use for them in research etc, asides from tormenting undergraduates?
I have been studying about the electronic spectra including the tanabe-sugano diagrams. My question is: in exercises where they give you the spectrum(a diagram which has the absorption and the wavelength) and they also give you the tanabe-sugano diagram and they ask you to assign transitions to bands how do we know for example that the band is due to the transition of two electrons or LMCT phenomenon or d-d transitions?Are there any tips? Do these exist? Where can I find them? I have only been able to find Tanabe-Sugano diagrams for octahedrals. I've been teaching my students about the Tanabe-Sugano diagram and what each individual term symbol represents in terms of the electronic structure for metal ions. For a d3 ion, there are three spin-allowed transitions from the 4A2g ground state - t2g^3 -: 4T2g <-- 4A2g 4T1g <-- 4A2g 4T1g (P) <-- 4A2g It's simple enough to deduce that the first two spin-allowed transitions corresponds to t2g(2) eg(1) <-- t2g(3), and that the last spin-allowed transition is t2g(1) eg(2)... I'm currently having trouble understanding Tanabe Sugano diagrams, especially when I'm asked to determine the transitions (T1g to T2g or something like that). Do you guys have any tips or things I should look out for when trying to determine these transitions?
**The following absorption bands are found in the spectrum of [Cr(CN)6]3-: 2640 angstrom, 3100 angstrom and 3780 angstrom. Calculate appropriate values of B' and Dq.** [Here's](https://upload.wikimedia.org/wikipedia/commons/thumb/c/c5/D3_Tanabe-Sugano_diagram.png/800px-D3_Tanabe-Sugano_diagram.png) the Tanabe-Sugano diagram for this system. The ground state for the d3 ion is 4A2g, so there are three spin-allowed transitions. However, I can't seem to find an x axis value where all the energy rat... The spectrum of [Ni(Metu)6]2+ (Metu = N-methylythiourea) has peaks of moderate intensity at 8800, 13,100 and 19,800 cm-1. Estimate the value of Dq and B.



















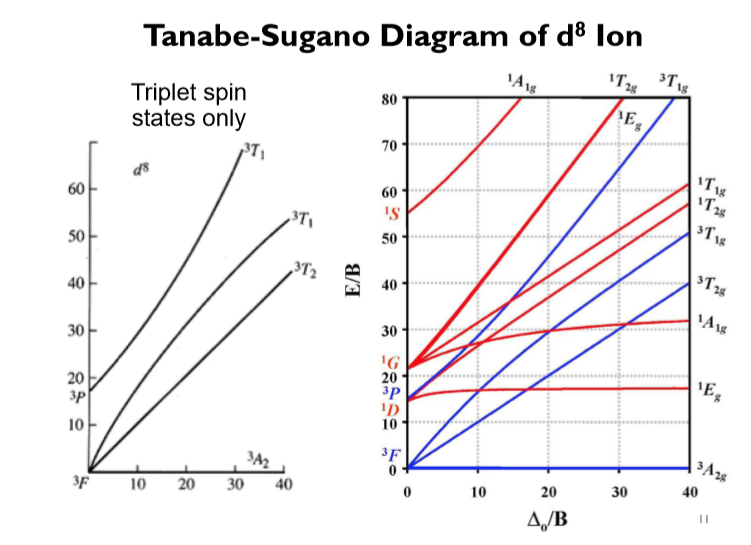



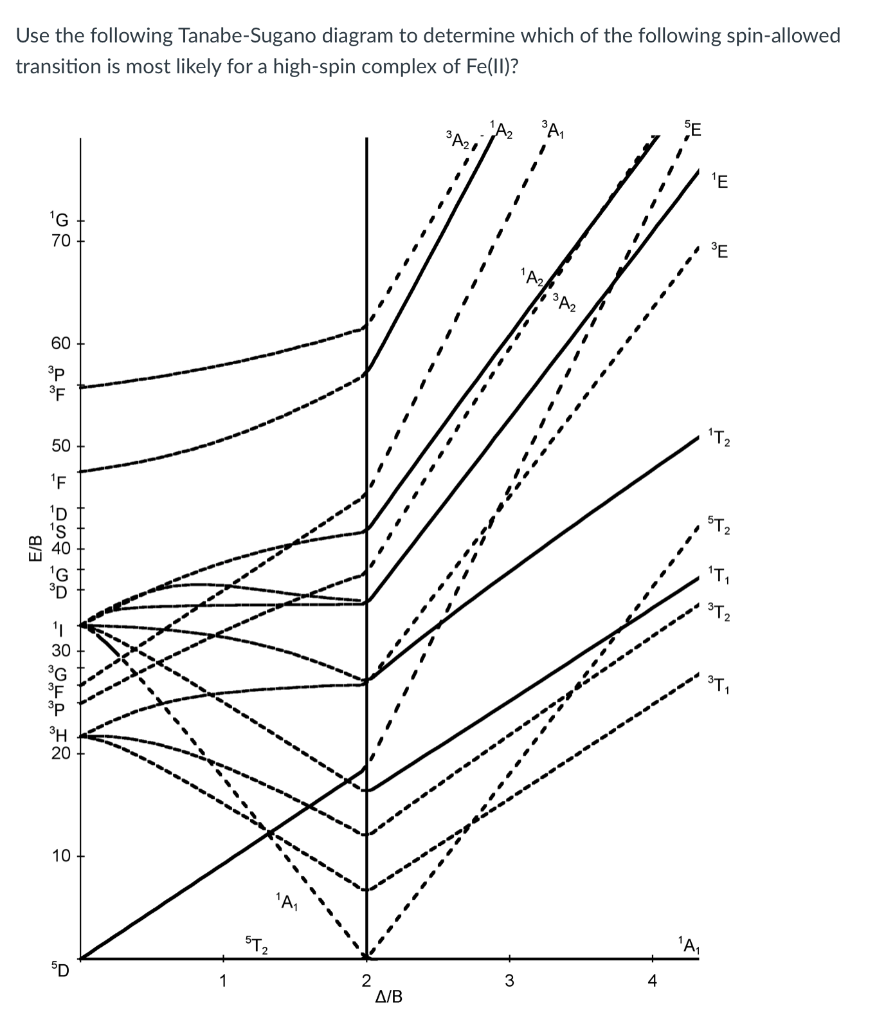
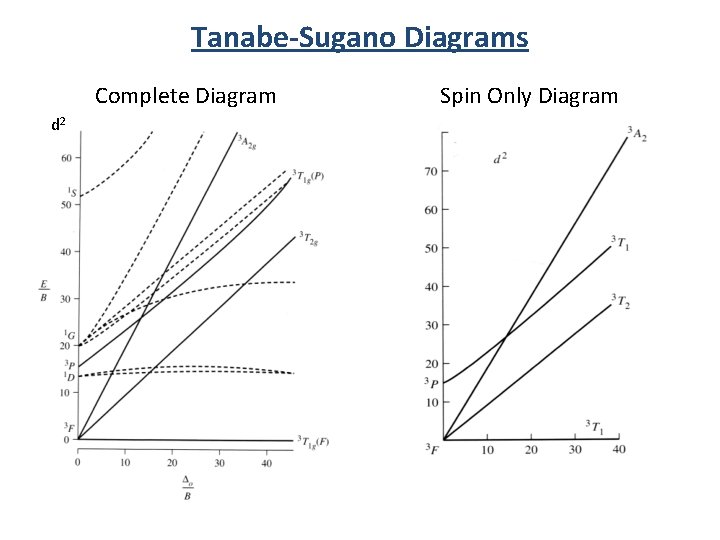


0 Response to "44 tanabe-sugano diagram"
Post a Comment