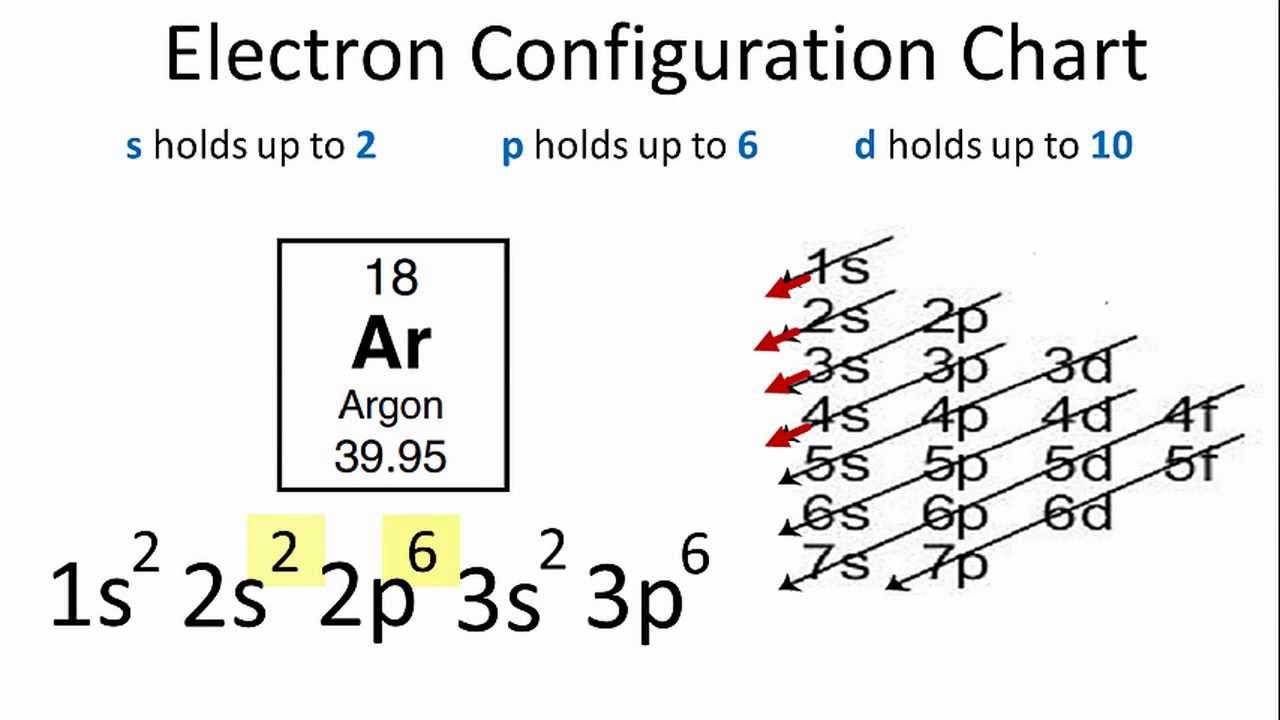
orbital diagram for argon
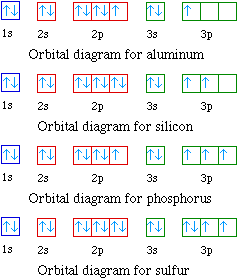
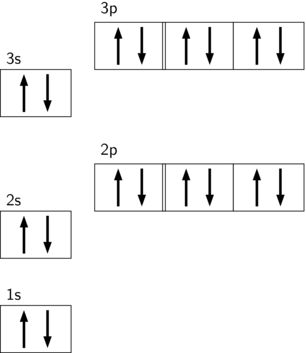
Argon(Ar) orbital diagram The next three electrons will enter the 2p orbital in the clockwise direction and the next three electrons will enter the 2p orbital in the anti-clockwise direction. The next two electrons will enter the 3s orbital and the next three electrons will enter the 3p orbital in the clockwise direction and the remaining three electrons will enter the 3p orbital in the anti-clockwise direction. What is the orbital diagram for Argon? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining six electrons.
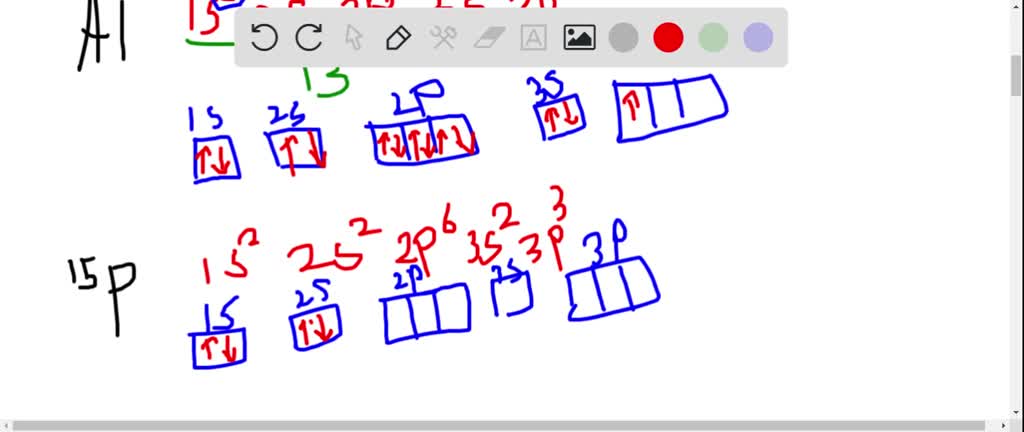
9. Draw the orbital diagram for: (follow the diagram on #8 above) Hint: it may help to write the electron configuration first. Nitrogen [Z=7] Oxygen (Z=8) Argon [Z=18] Potassium [Z=19] a. 1s2 2s2 2p5 b. 1s2 22s2 42p6 3s2 3p6 4s 3d

Orbital diagram for argon
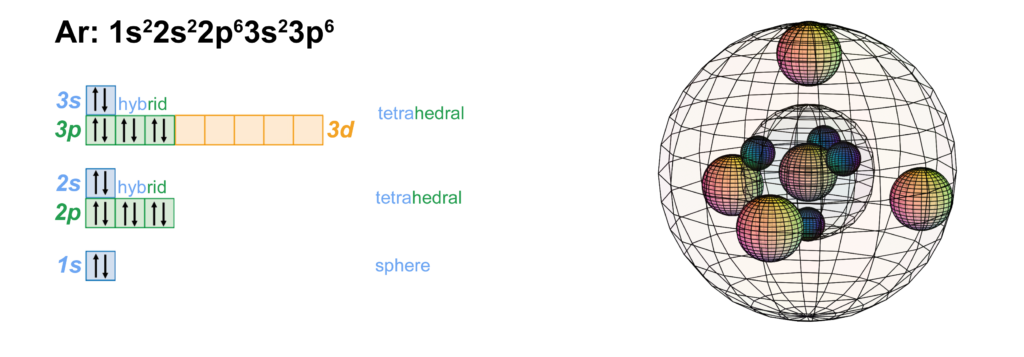
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Density: 0.00166 g/cm 3 . Electronic configuration of the Argon atom: 1s 2 2s 2 2p 6 3s 2 3p 6. Reduced electronic configuration Ar: [Ne] 3s 2 3p 6. Below is the electronic diagram of the Argon atom Distribution of electrons over energy levels in the Ar atom. 1-st level (K): 2. 2-st level (L): 8. 3-st level (M): 8. 23 Feb 2012 — Convert from orbital representation diagrams to electron ... Now, let's draw the electron configuration for Argon and place the electron ...
Orbital diagram for argon. Argon dimer. Formula: Ar 2. Molecular weight: 79.896. IUPAC Standard InChI: InChI=1S/2Ar. Copy Sheet of paper on top of another sheet. IUPAC Standard InChIKey: XMPZLAQHPIBDSO-UHFFFAOYSA-N. Copy Sheet of paper on top of another sheet. CAS Registry Number: 12595-59-4. Chemical structure: Write the complete orbital configuration for argon. Electronic Configuration: The electronic configuration of an atom shows the packing of electrons in the electron orbitals. Period 3 (Sodium to Argon) With sodium, we add a 3s orbital and at aluminum we begin adding 3p orbitals. Note that the 3p orbitals are larger than the 3s orbital. Argon is the next element with a stable octet with complete p and s orbitals. The reason the octet is so stable is that the only way to add electrons is to add the next s orbital outward. Orbital diagram. Tags: Question 12. SURVEY. 300 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices.
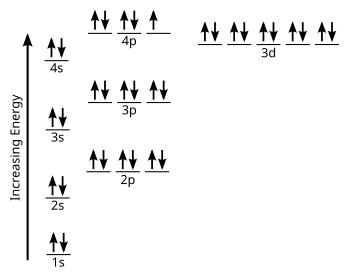
In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s22s22p3. What is the orbital diagram for arsenic? The electronic configuration of Argon (atomic number is 18) is-. 1s22s22p63s23p6. Note:- For writing the electronic configuration of ... Argon has completely filled M shell (or 3p orbital) What is an orbital diagram? An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total... We’re being asked to fill the orbital energy diagram for argon. Before we can do that, we have to first identify the number of electrons. • In a neutral atom: Atomic number = # of protons = # of electrons. Ar: atomic number = 18 → 18 protons & 18 electrons. • Distribute electrons in the atomic orbitals: 92% (245 ratings)
Orbital diagrams are pictorial descriptions of the electrons in an atom. Three rules are useful in forming orbital diagrams. According to the Auf Bau Principle, each electron occupies the lowest energy orbital. ... What is the orbital diagram for Argon? The p orbital can hold up to six electrons. 30 seconds. Q. What is incorrect about this orbital diagram? answer choices. Both arrows in the 2p box should be pointing up. There is nothing incorrect with this diagram. In the 2p box there should only be 1 electron in the first 2p box and one in the 2nd 2p box. All the arrows should be pointing up. Tags: Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ... 1s2 2s2 2p6 3s2 What element is represented by this orbital diagram?.In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. The next six electrons will go in the 2p orbital.
What is the orbital diagram for Argon? In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. Similar Asks
Chromium (Cr) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Fill the orbitals in this order 1s then 2s then 2p then 3s then 3p, from bottom to top on the orbital diagram. The chemical symbol for Argon is Ar. The idea is to draw an arrow for each electron, so in this case we just have one arrow to draw. It goes in the next box up, called the 2s orbital. Q.
Orbital diagram of Argon (Ar) 19: Orbital diagram of Potassium (K) 20: Orbital diagram of Calcium (Ca) 21: Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27:
This video shows how to create an orbital diagram of an atom from its electronic configuration
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration. (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) 1s. →. 2s.
The diagram below represents the orbital representation diagram used in earlier chapters. The orbital representation diagram has a few different variations but all are used to draw electron configurations. Most show the orbitals in groups, as lines, boxes, or circles with each orbital having its own line (or circle) within each sublevel.
In writing the electron configuration for Argon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Argon go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.
The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Argon (Ar) has an atomic mass of 18. Find out about its chemical and physical ... Orbital Diagram. Ar - Argon - Orbital Diagram - Electron Configuration ...
24 Jan 2021 — 1s only hold two electrons and the next 2 electrons for Argon goes in the 2s orbital. The next six electrons go to 2p orbital. The p orbital ...
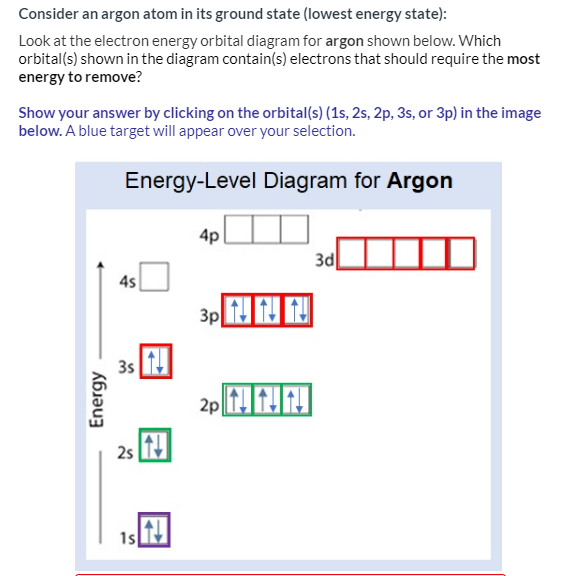
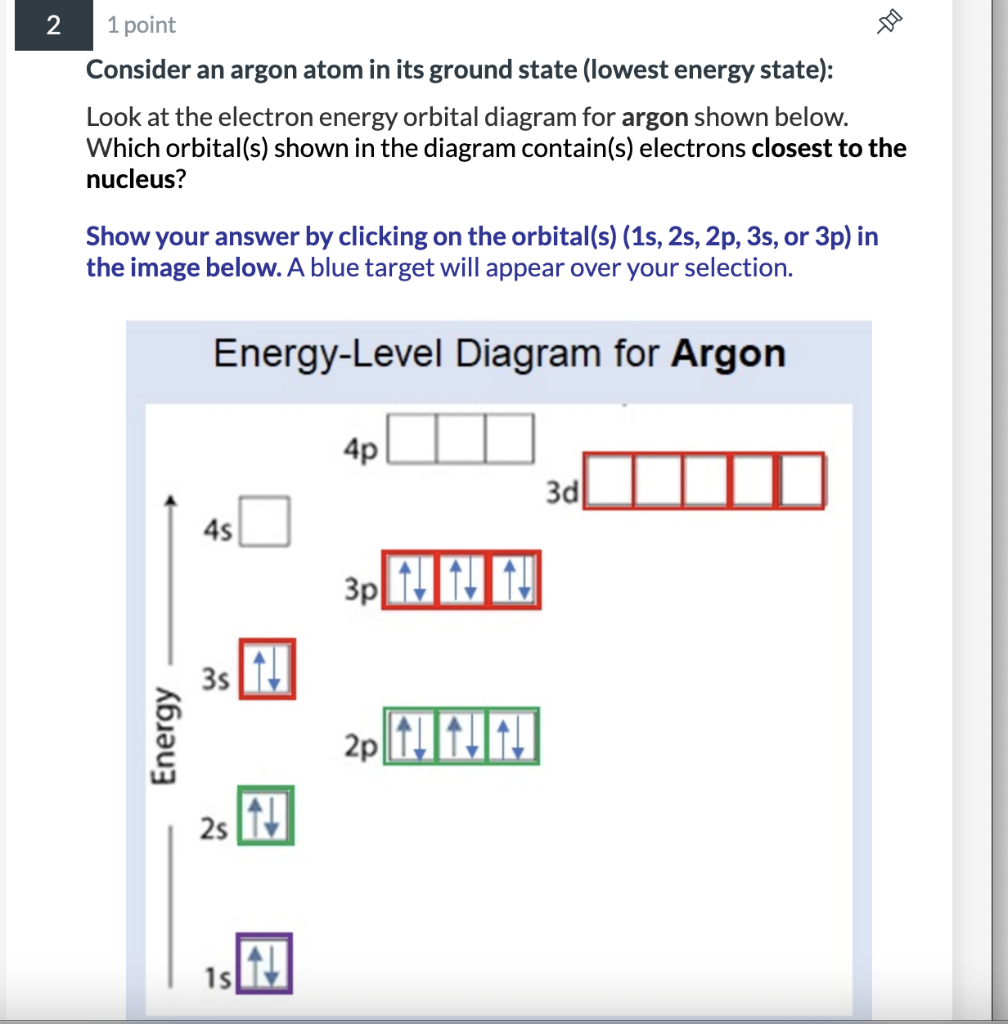
Chemistry. Chemistry questions and answers. Fill in the orbital energy diagram for argon. 3p 3s E 2p 2s བ ། 1s.
Krypton Orbital Diagram. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of krypton (atomic number: 36), the most common . Box spin diagram of outer electron orbitals for the electron configuration of the atom . 36 Krypton, Kr, [Ar]3ds24p6 = [Kr] (), [Ar]3d 4s 4p v. stable, Kr .
23 Feb 2012 — Convert from orbital representation diagrams to electron ... Now, let's draw the electron configuration for Argon and place the electron ...
Density: 0.00166 g/cm 3 . Electronic configuration of the Argon atom: 1s 2 2s 2 2p 6 3s 2 3p 6. Reduced electronic configuration Ar: [Ne] 3s 2 3p 6. Below is the electronic diagram of the Argon atom Distribution of electrons over energy levels in the Ar atom. 1-st level (K): 2. 2-st level (L): 8. 3-st level (M): 8.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.












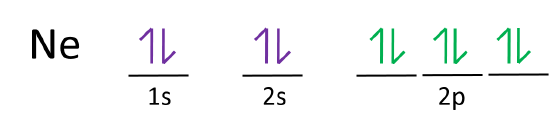















0 Response to "orbital diagram for argon"
Post a Comment