42 hydrogen energy level diagram
The energy level diagram for an hydrogen like atom is ... Click here👆to get an answer to your question ️ The energy level diagram for an hydrogen like atom is shown in the figure.The radius of its first Bohr orbit is 0 eV n = ∞ - 6.04 eV n = 3 - 13.6 eV n = 2 - 54.4 eV n = 1 Draw a neat labelled energy level diagram of the Hydrogen ... The following diagram shows the energy level diagram of the Hydrogen atom. A Hydrogen atom has only one single electron revolving around the nucleus at the lowest energy level. When we heat a hydrogen atom, the electron gains energy and moves up to higher energy levels.
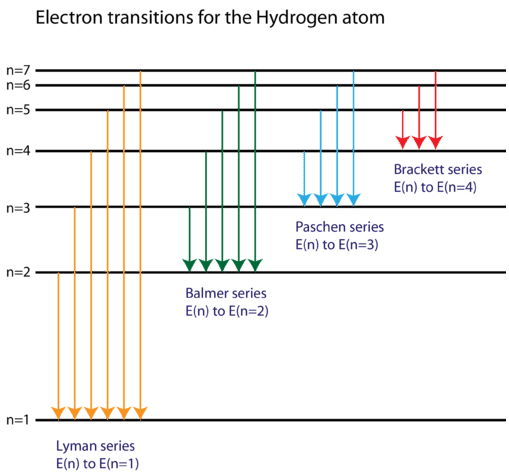
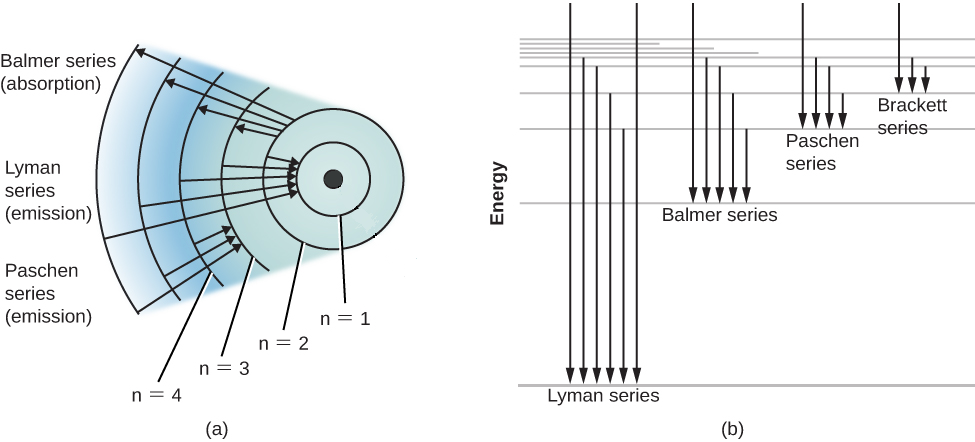
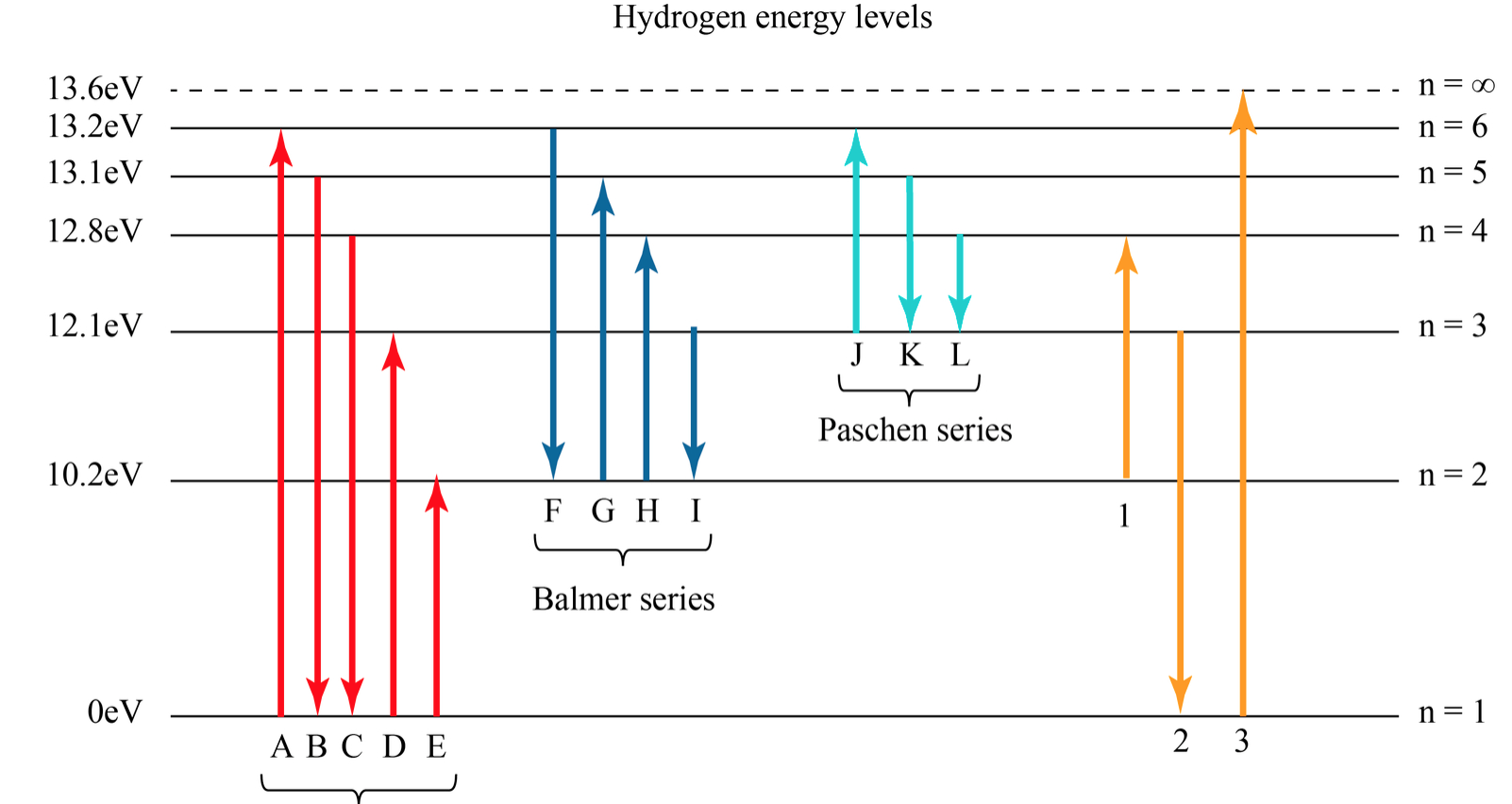
Hydrogen Spectrum - Diagram, Wavelength, Hydrogen Emission ... The Energy level diagram for the Hydrogen Atom indicates the different series of lines that is observed in the Spectrum. The horizontal lines witnessed refer to the different Energy levels. The vertical lines on the other hand refer to the transition that the Electron undergoes moving from the higher Energy level to the lower Energy level.

Hydrogen energy level diagram
Hydrogen(H) electron configuration and orbital diagram Hydrogen (H) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction. This is clearly shown in the figure of the orbital diagram of hydrogen. Diagram of energy levels of hydrogen lines | Physics Forums a) Draw a diagram of energy levels to explain the spectrum of lines of the hydrogen atom. b) Indicate, for each photon, that its region can be emitted to the electromagnetic spectrum. c) Compare, in a graph, the energies of the orbital of the hydrogen atom with the energies of the He +. Negative energy levels in the diagram for a hydrogen atom Negative energy levels in the diagram for a hydrogen atom. Ask Question Asked 7 years, 6 months ago. Modified 4 years, 11 months ago. Viewed 40k times 12 7 $\begingroup$ The higher the number of the shell (n), the higher is the energy level of the electron. However, why was it necessary to have negative values.
Hydrogen energy level diagram. What is energy level diagram of hydrogen atom ... Hint: Energy level diagram is the direct consequence of the principal quantum number - 'n'. A Hydrogen atom has only one single electron revolving around the nucleus at the lowest energy level. When we heat a hydrogen atom, the electron gains energy and moves up to higher energy levels. Hydrogen Spectrum - Balmer Series, Definition, Diagram ... Hydrogen Spectrum - Wavelength, Diagram, Hydrogen Emission Spectrum. Hydrogen Spectrum introduction. We all know that electrons in an atom or a molecule absorb energy and get excited, they jump from a lower energy level to a higher energy level, and they emit radiation when they come back to their original states. Draw Energy Level Diagram for Hydrogen Atom - Hyder Thatirly66 Mar 07, 2022 · The electron in the hydrogen atom is bars in the potential well, and its total energy is negative. The energy levels in a hydrogen atom can be obtained past solving Schr dinger s equation in three dimensions. We have to solve the radial equation (-ħ two / (2m))∂ 2 (rR))/∂r 2 + (l (l + 1)ħ 2 / (2mr 2 )) (rR) - (E - e 2 /r) (rR) = 0 or hydrogen atom energy level diagram - Energy Level Diagram Dec 04, 2021 · The energy level you see is the number of empty energy levels of hydrogen. The atoms that make up hydrogen are the very first elements to be discovered in the universe. They were discovered in 1907 by two German scientists, Otto Hahn and Fritz Haber, at the Kaiser Wilhelm Institute in Berlin, under the supervision of a professor named Niels Bohr.
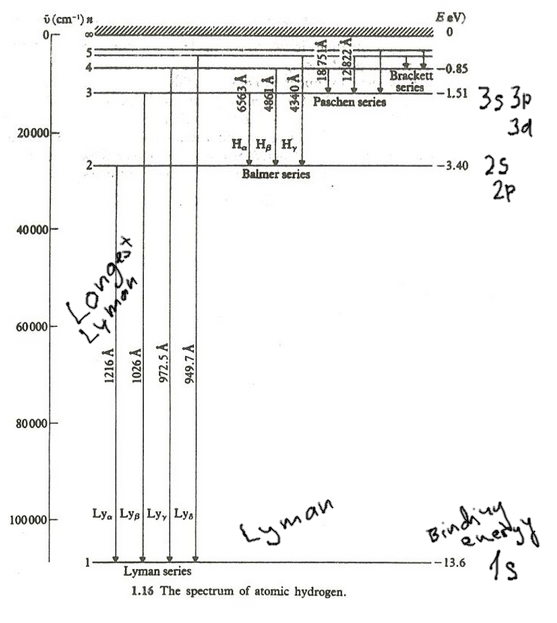
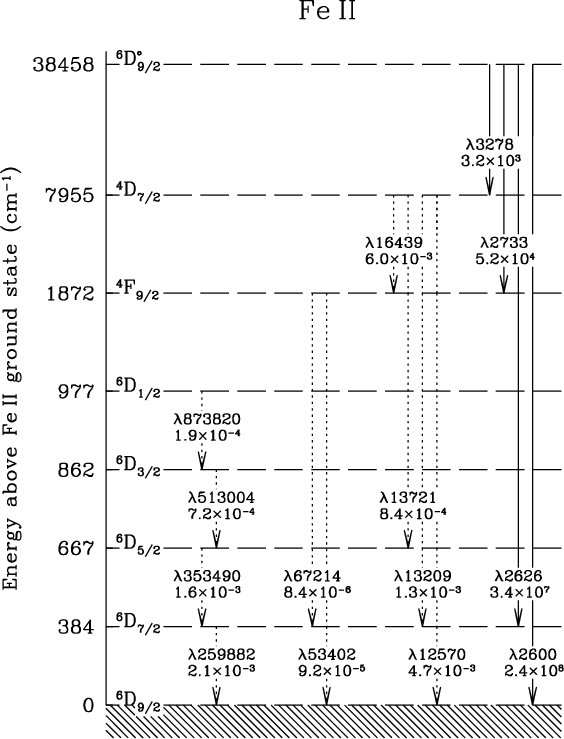
Hydrogen Energy Levels - Georgia State University The three prominent hydrogen lines are shown at the right of the image through a 600 lines/mm diffraction grating. An approximate classification of spectral colors: Violet (380-435nm) Blue(435-500 nm) Cyan (500-520 nm) Green (520-565 nm) Yellow (565- 590 nm) Orange (590-625 nm) Red (625-740 nm) Hydrogen molecule, energy level diagram - Big Chemical ... Energy level diagram for hydrogen molecule, H2, and separated atoms H R = 00) and He R = 0). R = the Rydberg constant = 13.6057 eV = 0.5 a.u. (atomic unit of energy). Value from ionization potential of He (Is 2p P). Value from ionization potential of H2. The experimental ionization potentials are quite precise but for systems containing more ... Why do energy level diagrams differ for hydrogen and ... Answer (1 of 3): Hydrogen has only one electron, so the only relevant force (in a classical approximation) is between the one negatively charged electron and the positively charged nucleus. In a multi-electron atom, the negatively charged electrons repel each other. We call this effect "electron... Solved Use the hydrogen energy level diagram provided to ... Hydrogen Energy Level DiagramThe orbitals of hydrogen from n=1 to n=6. The energy level of each orbital and the ionization energy are provided on the left of the diagram. Various transitions of electrons between the orbitals, resulting from the emission and absorption of photons of different energies for the Lyman, Balmer, and Paschen ...
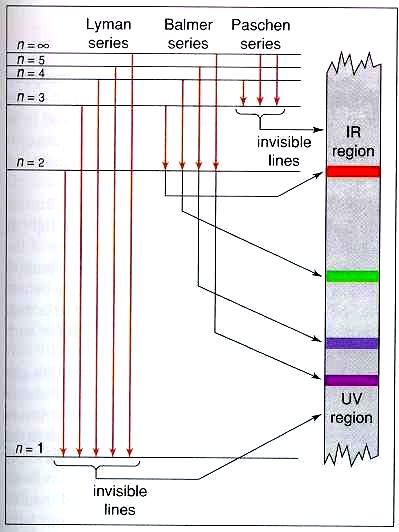
Energy Level and Transition of Electrons | Brilliant Math ... Imgur. The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The energy level of the electron of a hydrogen atom is given by the following formula, where. n. n n denotes the principal quantum number: E n = − 1312 n 2 kJ/mol. E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n. Draw a Neat and Labelled Energy Level Diagram and Explain ... Draw a Neat and Labelled Energy Level Diagram and Explain Balmer Series and Brackett Series of Spectral Lines for Hydrogen Atom. Maharashtra State Board HSC Science (General) 12th Board Exam. Question Papers 255. Textbook Solutions 14283. MCQ Online Tests 73. ... L23.1 Energy levels and diagram for hydrogen. - YouTube MIT 8.04 Quantum Physics I, Spring 2016View the complete course: : Barton ZwiebachLicense: Creative Commons BY-NC-SAMore ... Energy level diagrams and the hydrogen atom - Boston University Energy level diagrams and the hydrogen atom It's often helpful to draw a diagram showing the energy levels for the particular element you're interested in. The diagram for hydrogen is shown above. The n = 1 state is known as the ground state, while higher n states are known as excited states.
Hydrogen Energy Level Diagram – GeoGebra Hydrogen Energy Level Diagram. New Resources. Kopie von Break-Even-Analyse; Equations of Perpendicular Lines (Open Middle) - V2
Solved 14. Which of the transitions in the hydrogen atom ... Which of the transitions in the hydrogen atom energy-level diagram shown here requires the absorption of the longest wavelength photon? transition a b. ransition b c transition c d. ransition d n 5 a. e. None of these are correct, because photons are emitted, not absorbed, by these transitions 15.
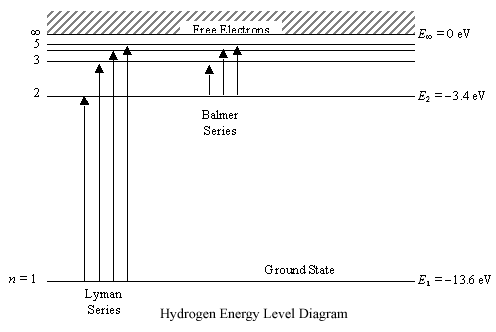
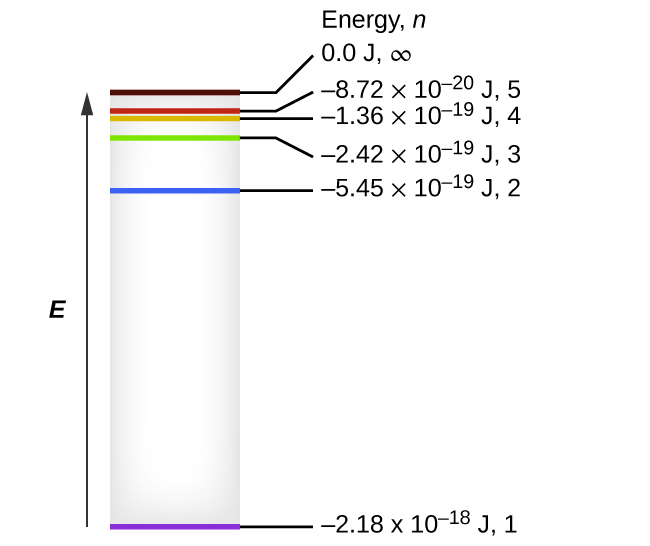
What is energy level diagram for hydrogen atom ... The electron energy level diagram for the hydrogen atom. He found that the four visible spectral lines corresponded to transitions from higher energy levels down to the second energy level (n=2). This is called the Balmer series.
Chapter 7 CHM 151 Flashcards - Quizlet Which of the transitions in the following hydrogen atom energy level diagram involves the shortest wavelength photon? A. Which transition in a hydrogen atom will cause emission of the shortest wavelength photon. N1 = 3, n2 = 1. Which combination of quantum numbers is possible for an atom with five orbitals in one subshell.
Energy Level Diagram For Hydrogen - Mini Physics Nov 21, 2010 · For hydrogen, the ionization energy = 13.6eV When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon. The Lyman (ultraviolet) series of spectral lines corresponds to electron transitions from higher energy levels to level n = 1.
Draw a Neat, Labelled Energy Level Diagram for H Atom ... Draw a Neat, Labelled Energy Level Diagram for H Atom Showing the Transitions. Explain the Series of Spectral Lines for H Atom, Whose Fixed Inner Orbit Numbers Are 3 and 4 Respectively.
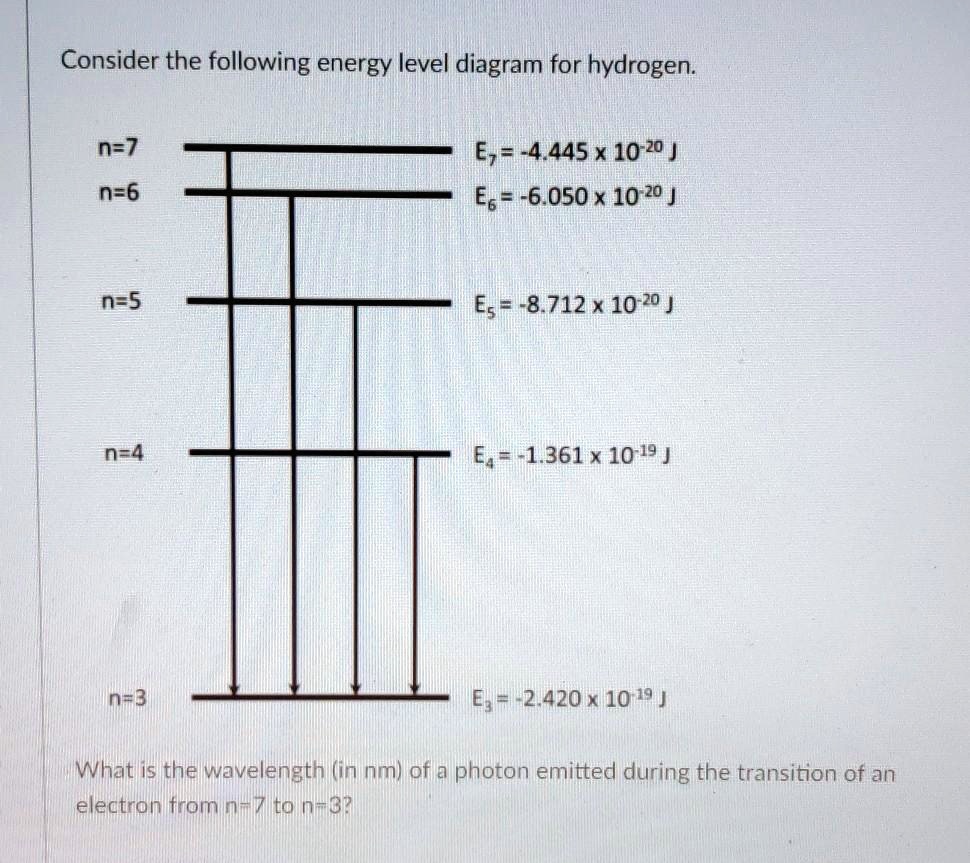
The figure shows energy level diagram of hydrogen atom. (i ... The figure shows energy level diagram of hydrogen atom. (i) Find out the transition which results in the emission of a photon of wavelength 496 nm. (ii) Which transition corresponds to the emission of radiation of maximum wavelength ? Justify your answer.
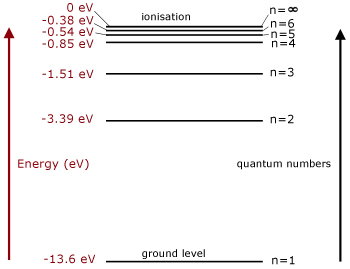
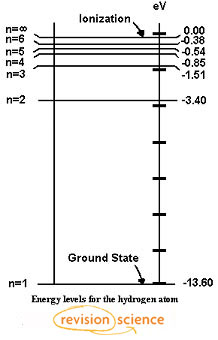
Spectral series of hydrogen atom and Energy level diagram Energy level diagram The energy of the electron in the nth orbit of the hydrogen atom is given by, En = -13.6 /n2 eV Energy associated with the first orbit of the hydrogen atom is, E1 = -13.6 /12 = -13.6 eV It is called ground state energy of the hydrogen atom. Energy associated with the second orbit is given by, E2 = -13.6 /22 = -3.4 eV
Draw a neat, labelled energy level diagram for the ... According to Bohr's model of the hydrogen atom, an atom exists most of the time in one of a number of stable and discrete energy states. The various states arranged in order of increasing energy constitute the energy level diagram of the atom, as shown in below figure for the hydrogen atom.
Partial Energy Level Diagram for Hydrogen - Partial Energy ... View Lab Report - Partial Energy Level Diagram for Hydrogen from CHEM 2070 at Cornell University. Partial Energy Level Diagram for Hydrogen 1 = (6.626 x 10-37 KJ) (3.00 x 1017 nm/s) (6.022 x 1023
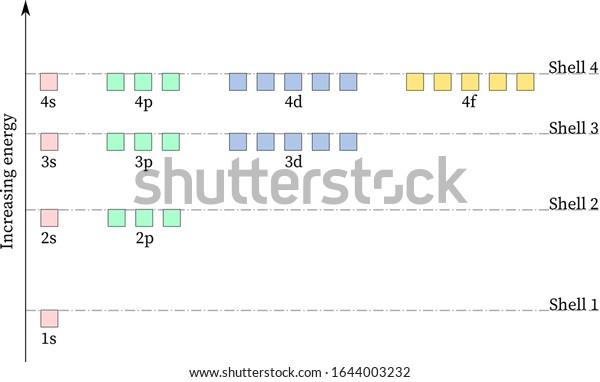
Energy Level Diagram - Different Energy Shells Around the ... What is energy level diagram? In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. The closest shell to the nucleus is called the "K shell" followed by the "L shell" then the "M shell" and so on away from the nucleus. The shells can be denoted by alphabets (K, L ...
Negative energy levels in the diagram for a hydrogen atom Negative energy levels in the diagram for a hydrogen atom. Ask Question Asked 7 years, 6 months ago. Modified 4 years, 11 months ago. Viewed 40k times 12 7 $\begingroup$ The higher the number of the shell (n), the higher is the energy level of the electron. However, why was it necessary to have negative values.
Diagram of energy levels of hydrogen lines | Physics Forums a) Draw a diagram of energy levels to explain the spectrum of lines of the hydrogen atom. b) Indicate, for each photon, that its region can be emitted to the electromagnetic spectrum. c) Compare, in a graph, the energies of the orbital of the hydrogen atom with the energies of the He +.
Hydrogen(H) electron configuration and orbital diagram Hydrogen (H) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction. This is clearly shown in the figure of the orbital diagram of hydrogen.























0 Response to "42 hydrogen energy level diagram"
Post a Comment