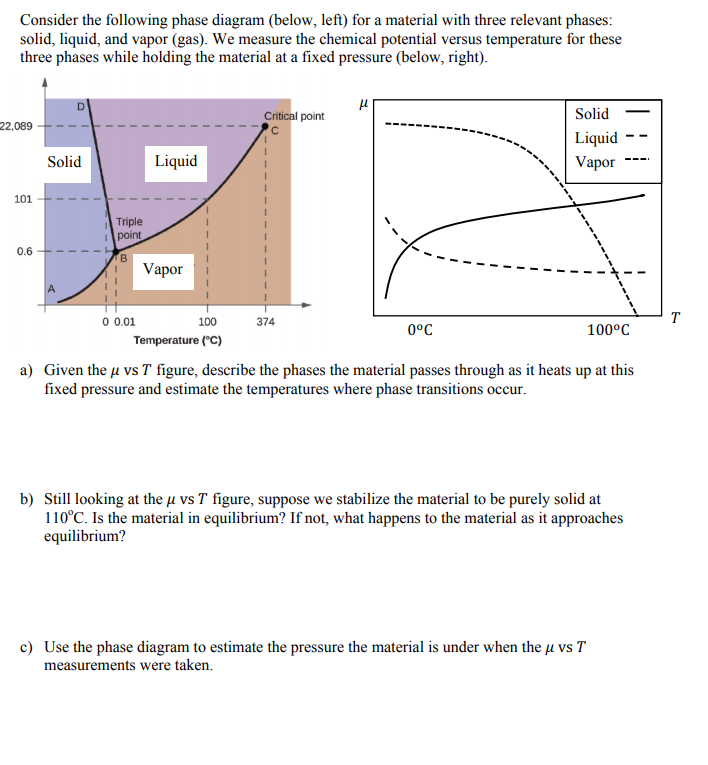
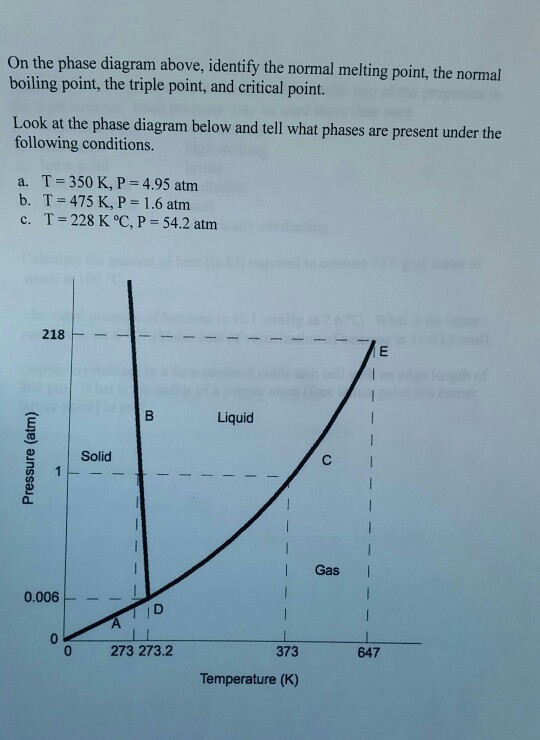
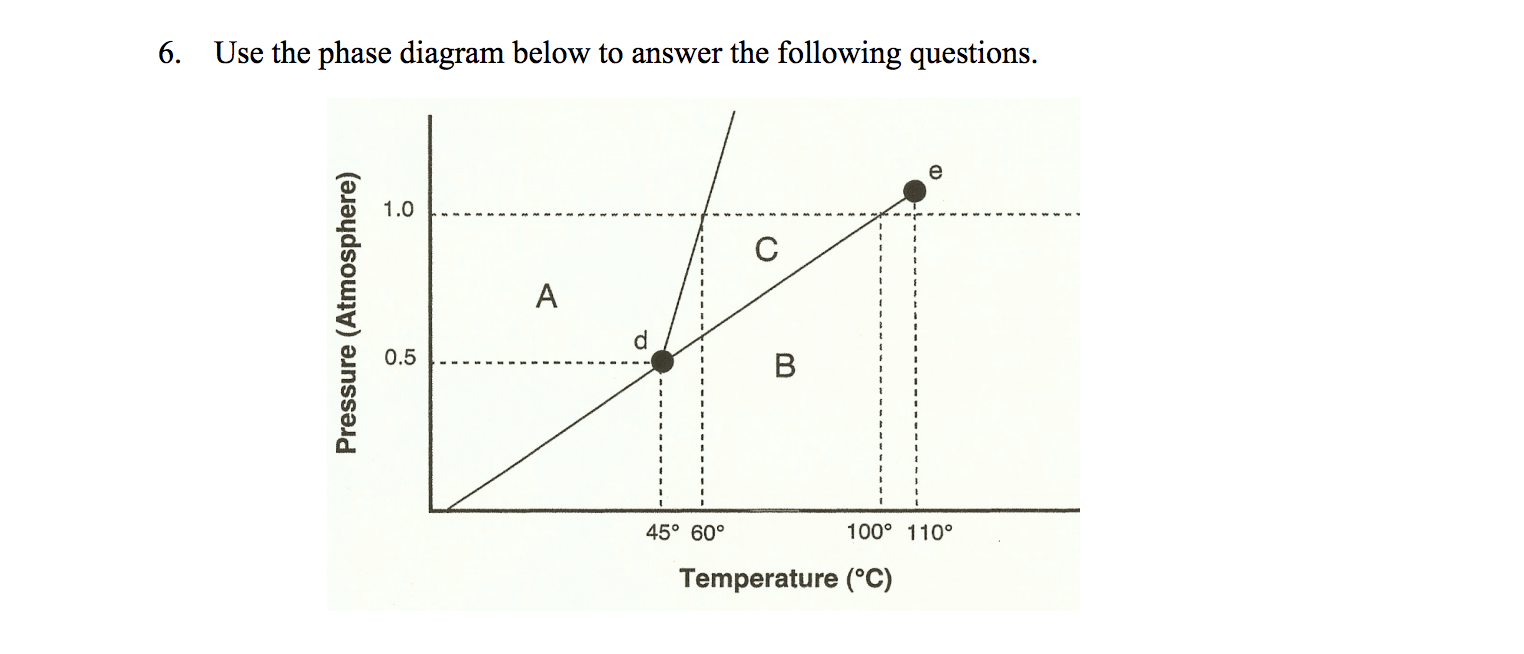
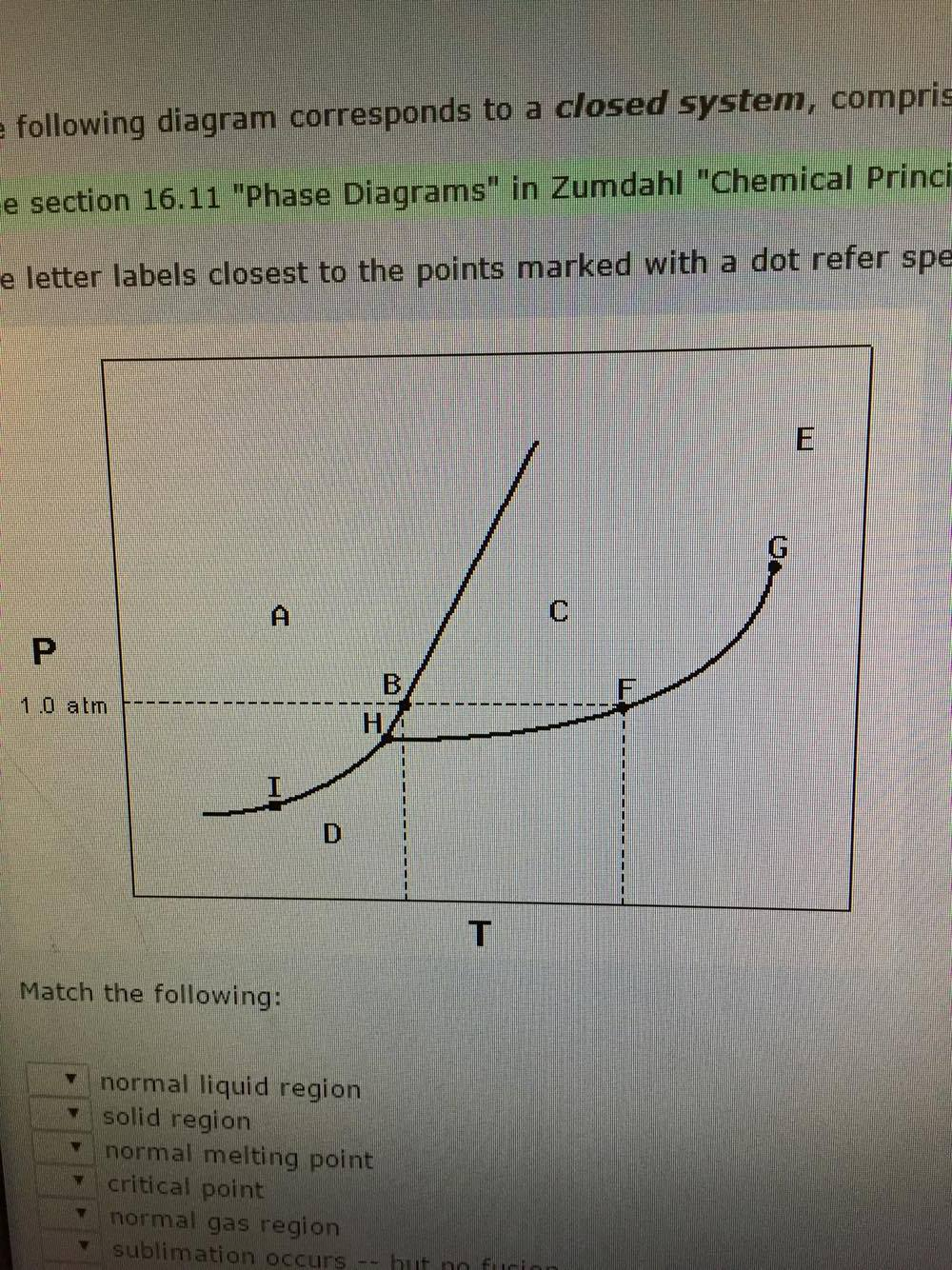
43 examine the following phase diagram and determine what phase exists at point d.
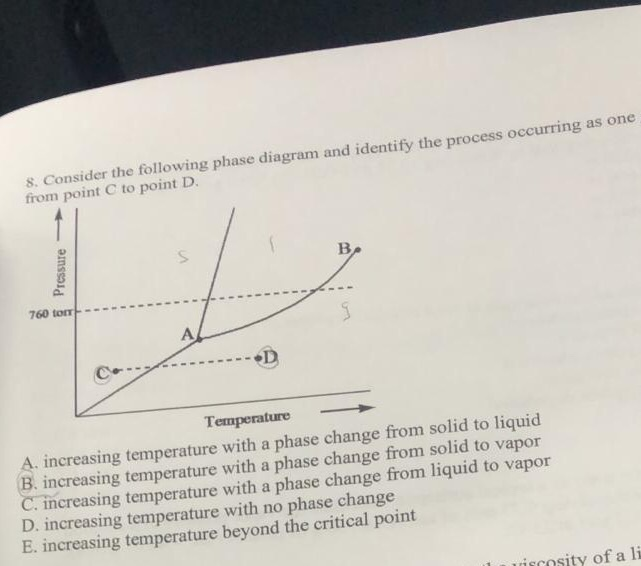
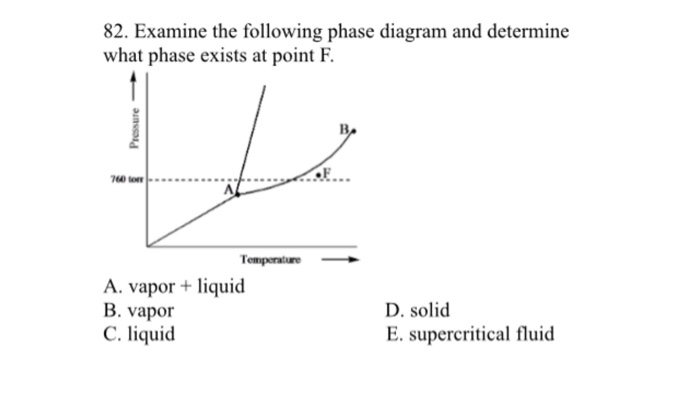
Examine the following phase diagram and determine what ... Examine the following phase diagram and determine what phase exists at point F. Previous Fill in each blank with the standard form of be, have, or do. Like me, the car ________ a problem dealing with freezing weather. Next Underline the standard verb form. Dan (climb, climbed) up on the roof to see where the water was coming in. Solved 12) Examine the following phase diagram and ... Question. : 12) Examine the following phase diagram and identify the feature represented by point A. 760 torr Temperature A) melting point B) critical point C) triple point D) sublimation point E) boiling point 13) In hydrogen iodide are the most important intermolecular forces A) B) C) dipole-dipole forces London dispersion forces hydrogen ...
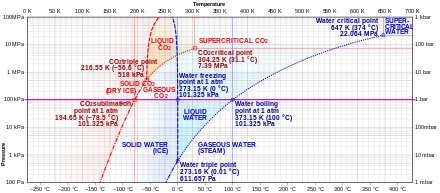
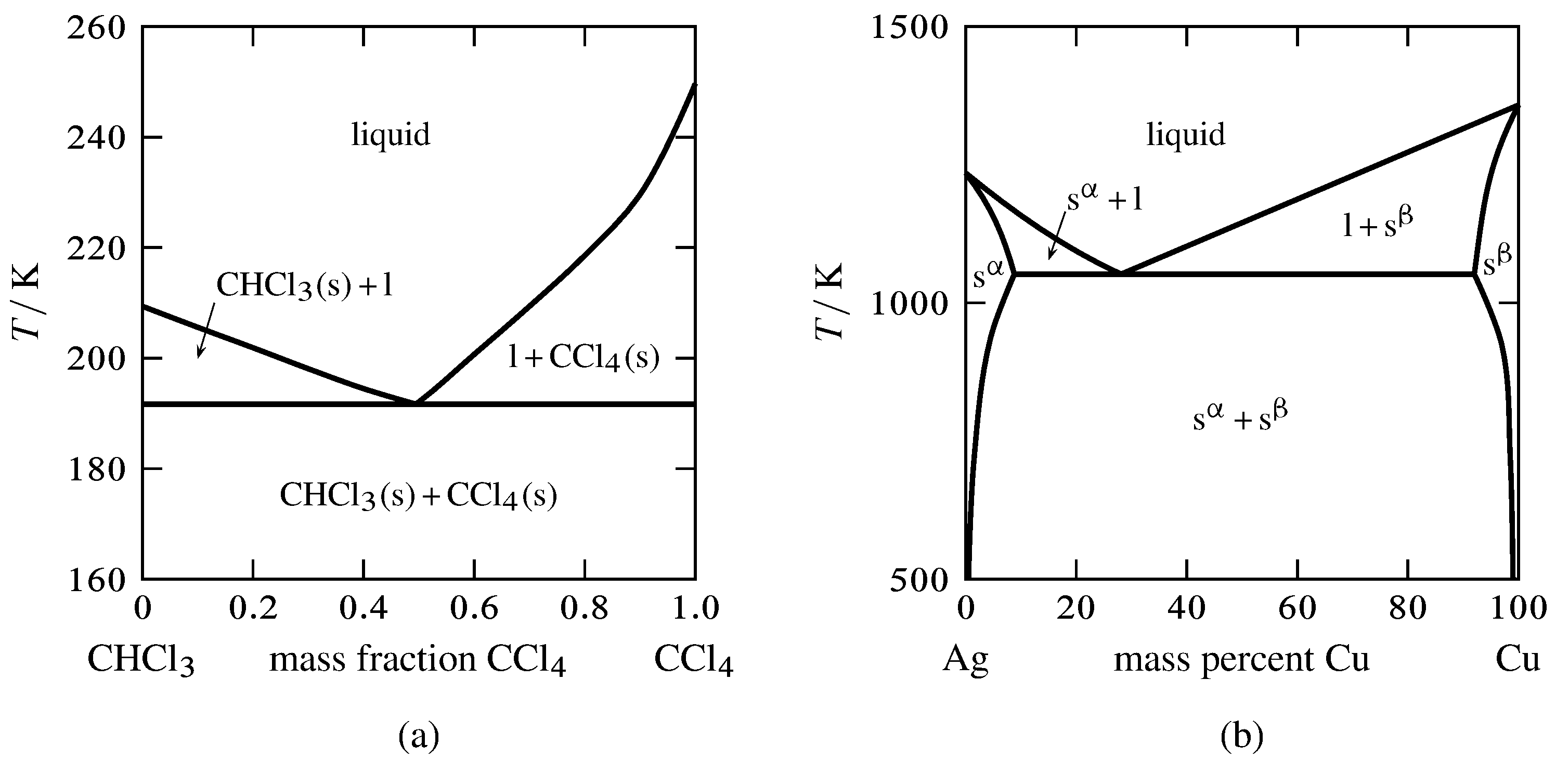
Features of Phase Diagrams (M11Q1) - UW-Madison Chemistry ... Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...

Examine the following phase diagram and determine what phase exists at point d.
Examine the following phase diagram and identify the ... Examine the following phase diagram and identify the feature represented by point B. A B 760 torr Temperature. A ) melting point. D ) sublimation point. B ) triple point. E ) boiling point. C ) critical point. 16 . Consider the following phase diagram and identify the process occurring as one goes from point C to point D. PDF CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS ε and phases ... This problem asks that we determine the phase mass fractions for the alloys and temperatures in Problem 9.8. (a) From Problem 9.8a, ε and η phases are present for a 90 wt% Zn-10 wt% Cu alloy at 400 °C, as represented in the portion of the Cu-Zn phase diagram shown below (at point A). G3BP1 Is a Tunable Switch that Triggers Phase Separation to ... Apr 16, 2020 · After 30 min of exposure, 250 nM of D/D solubilizer was added and cells were further incubated for 30 min. Images show live-cell imaging prior to and within 2 min after addition of D/D solubilizer. SG dissolution following treatment with D/D solubilizer was assessed by measuring the intensity of the SGs on the frame immediately before and after ...
Examine the following phase diagram and determine what phase exists at point d.. PPTP Client Feb 05, 2010 · You can examine the debug log to determine how this negotiation proceeded. Solution: choose one: provide an IP address on the command line, (see man pppd in the section marked OPTIONS, or just add the IP address followed by a colon, e.g. 10.0.0.1:), change the peer configuration to suggest a local IP address, Best Chemistry 02 chapter 10/11/12 Terms Flashcards - Quizlet Examine the following phase diagram and identify the feature represented by point A. ... Consider the following phase diagram and identify the process occurring as one goes from point C to point D. ... Examine the following phase diagram and determine what phase exists at point F. SEE QUESTION 14 Image A) vapor + liquid B) vapor Rahman Test 6 Chem 1311 Flashcards | Quizlet Examine the phase diagram for the substance Bogusium (Bo) and select the correct statement. Bo(s) has a lower density then Bo(l). The triple point for Bo is at a higher temperature than the melting point for Bo. Bo changes from a solid to a liquid as one follows the line from C to D. Point B represents the critical temperature and pressure for Bo. PDF Chapter 9: Phase Diagrams - Florida International University Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Solved Examine the following phase diagram and determine ... Question: Examine the following phase diagram and determine what phase exists at point 760 som Temperature A) supercritical fluid B) liquid C) vapor+liquid D) vapor E) solid The phase diagram of a substance i s given below. This substance is a at 25°C and 1.0 atm. 1.5 P (atm) 1.0 0.5T -10 0 10 20 30 40 50 60 70 T ('C) A) gas B) crystal C ... CHEM Exam 6 Flashcards | Quizlet 5) The phase diagram for xenon has a solid- liquid curve with a positive slope. Which of the following is true? A) Solid xenon has a higher density than liquid xenon. B) Solid xenon has the same density as liquid xenon. C) The phase diagram cannot be used to predict which phase of xenon is denser. D) Freezing xenon is an endothermic process. Chemistry Chapter 12 Flashcards - Quizlet Examine the following phase diagram and identify the feature represented by point B A) melting point B) triple point ... Examine the following phase diagram and determine what phase exists at point F. A) vapor+liquid B) vapor C) liquid D) solid E) supercritical fluid. B. ... The maximum number of phases of a single substance which can coexist ... HQ 12 - Chapter 12 -Consider the following phase diagram ... Chapter 12-Consider the following phase diagram and identify the process occurring as one goes from point C to point D. Increasing temperature with a phase change from solid to vapor -Examine the following phase diagram and determine what phase exists at point F.
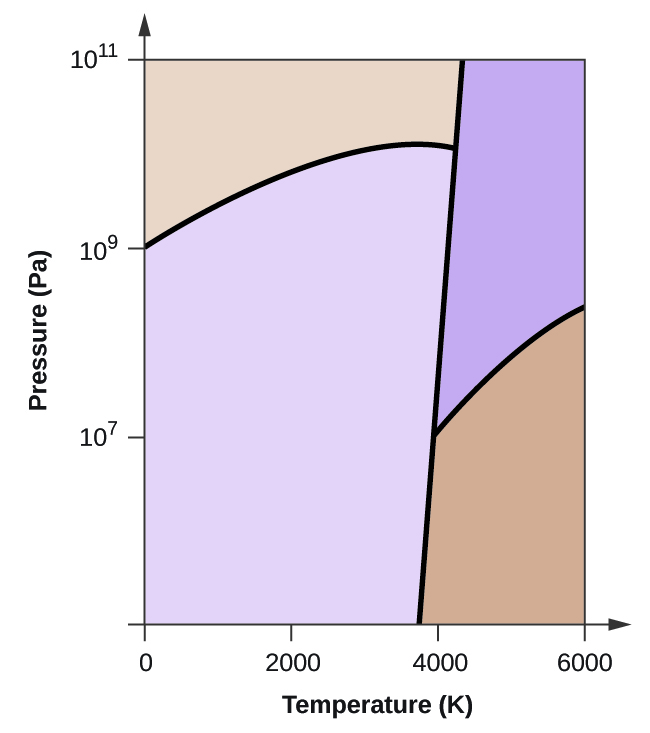
HOMEWORK 1 (work & answers).docx - HOMEWORK 1 Multiple ... View HOMEWORK 1 (work & answers).docx from CHEM 1122 at Gateway Community College. HOMEWORK 1 Multiple choice questions (4 points each) 1. Examine the following phase diagram and identify the feature Phase Diagrams | Chemistry for Majors - Lumen Learning Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ... Phase Diagrams - Chemistry - University of Hawaiʻi On the phase diagram, label the graphite phase. (c) If graphite at normal conditions is heated to 2500 K while the pressure is increased to 10 10 Pa, it is converted into diamond. Label the diamond phase. (d) Circle each triple point on the phase diagram. (e) In what phase does carbon exist at 5000 K and 10 8 Pa? PDF Assignment 7 solutions - University of California, San Diego As may be noted, point C lies within the Liquid phase field. Therefore, only the liquid phase is present; its composition is 55 wt% Ag-45 wt% Cu. (d) The Mg-Pb phase diagram (Figure 9.20) is shown below; the point labeled "D" represents the 30 wt% Pb-70 wt% Mg composition at 425°C.
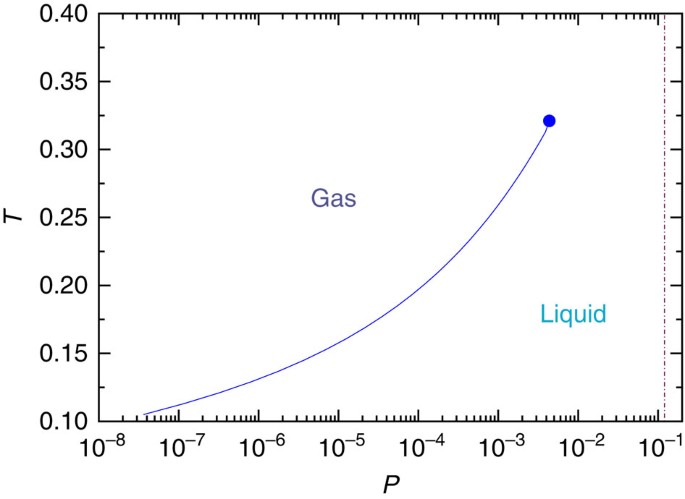
Phase Diagram for Water | Chemistry for Non-Majors Phase Diagram for Water. Water is a unique substance in many ways. One of these special properties is the fact that solid water (ice) is less dense than liquid water just above the freezing point. The phase diagram for water is shown in the Figure below .
Phase Diagrams | Boundless Chemistry - Lumen Learning Phase Diagram: In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries.The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the conditions under which a solid can be converted directly to a gas (and vice-versa).
Chapter 10/11/12 Terms Flashcards - Quizlet Examine the following phase diagram and identify the feature represented by point A. ... Consider the following phase diagram and identify the process occurring as one goes from point C to point D. ... Examine the following phase diagram and determine what phase exists at point F. SEE QUESTION 14 Image A) vapor + liquid B) vapor
5 System Design for Reliability | Reliability Growth ... throughout the life of the product with low overall life-cycle costs. The techniques that comprise design for reliability include (1) failure modes and effects analysis, (2) robust parameter design, (3) block diagrams and fault tree analyses, (4) physics-of-failure methods, (5) simulation methods, and (6) root-cause analysis.
Coursework Hero - We provide solutions to students From Literature to Law – we have MA and Ph.D. experts in almost any academic discipline, for any task. Any Paper We can write, proofread, paraphrase, format, edit or rewrite your any paper, whether it’s a review or a term paper.
Solved Examine the following phase diagram and determine ... Examine the following phase diagram and determine what phase exists at point C. 1. gas and liquid 2. gas 3. liquid 4. solid 5. supercritical fluid; Question: Examine the following phase diagram and determine what phase exists at point C. 1. gas and liquid 2. gas 3. liquid 4. solid 5. supercritical fluid
10.4 Phase Diagrams - Chemistry Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
Phase Diagrams - Phases of Matter and Phase Transitions These phases exist in equilibrium with one another. There are two points of interest on a phase diagram. Point D is the point where all three phases meet. When the material is at this pressure and temperature, it can exist in all three phases. This point is called the triple point.
Ternary Phase Diagram - an overview | ScienceDirect Topics A point having composition (A, B, C) on Figure 4.23 can be located anywhere on the ternary diagram and such a point represents the overall composition of the phase, regardless of the number of phases. The compositions on a ternary diagram must sum to unity if in mole or weight fractions, or to 100 if in percent.
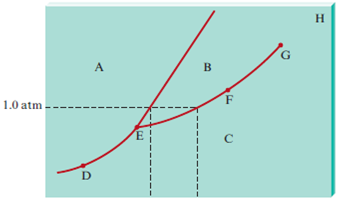
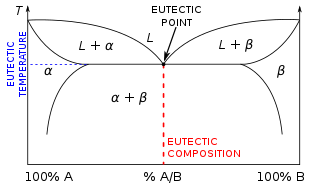
2 Component Phase Diagrams - Tulane University Eutectic point - the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature must remain constant until one of the phases disappears. A eutectic is an invariant point. Peritectic point - The point on a phase diagram where a reaction takes place between a previously ...
- movebox.vc . Coming soon. ... . Coming soon.
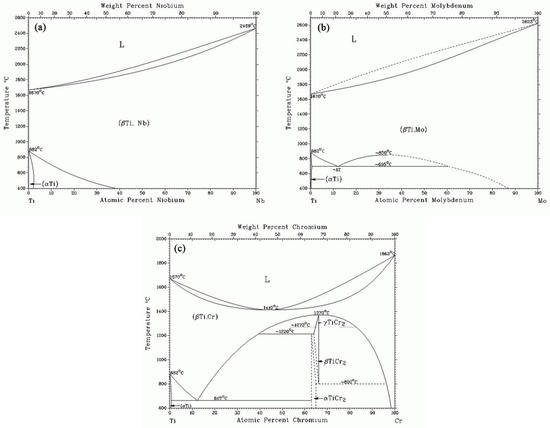
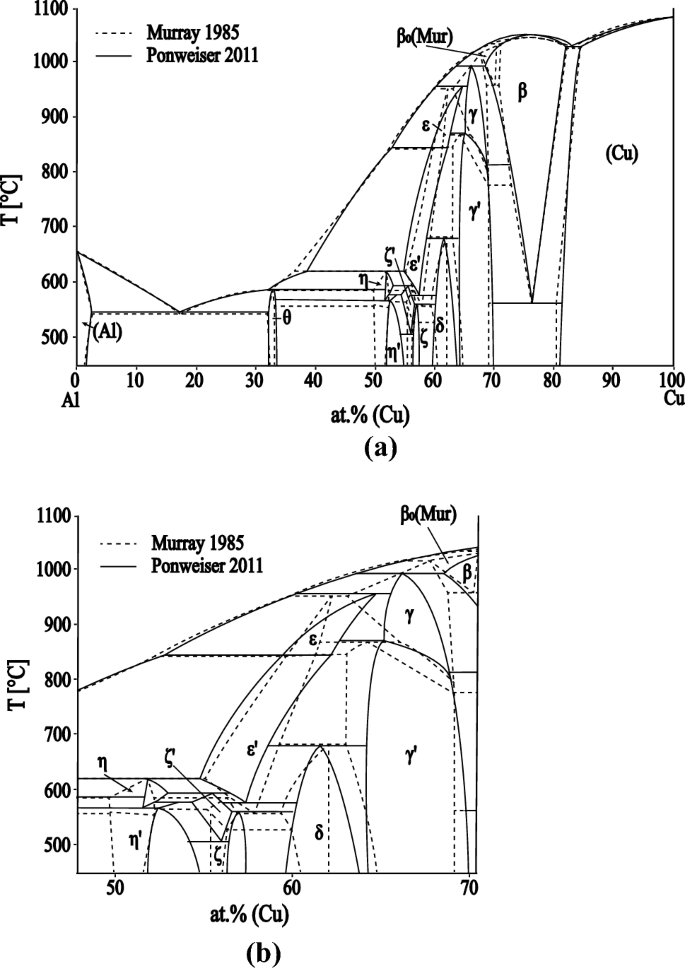
The Iron-Carbon Phase Diagram - IspatGuru The Iron-Carbon Phase Diagram. The phase diagrams are very important tools in the study of alloys for solutions of many practical problems in metallurgy. These diagrams define the regions of the stability of a phase which can exist in an alloy system under the condition of constant atmospheric pressure. For a binary system, the coordinates of ...
PDF 1331 - Chapter 11 Questions Ignore Questions 3, 10, 37 ... (A) 10.1 kJ (B) 13.1 kJ (C) 16.1 kJ (D) 48.6 kJ 38. Examine the following phase diagram and identify the feature represented by point A. A. melting point B. critical point C. triple point D. sublimation point 39. What are the changes in phase going from points A to B to C to D A B D C T P
PDF Lecture 19: 11.23.05 Binary phase diagrams 3-Dimensional Depiction of Temperature-Composition Phase Diagram of Bismuth, Tin, and Lead at 1atm. The diagram has been simplified by omission of the regions of solid solubility. Each face of the triangular a eutectic. There is also a peritectic point in the Bi-Pb phase diagram. Figure by MIT OCW.
G3BP1 Is a Tunable Switch that Triggers Phase Separation to ... Apr 16, 2020 · After 30 min of exposure, 250 nM of D/D solubilizer was added and cells were further incubated for 30 min. Images show live-cell imaging prior to and within 2 min after addition of D/D solubilizer. SG dissolution following treatment with D/D solubilizer was assessed by measuring the intensity of the SGs on the frame immediately before and after ...
PDF CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS ε and phases ... This problem asks that we determine the phase mass fractions for the alloys and temperatures in Problem 9.8. (a) From Problem 9.8a, ε and η phases are present for a 90 wt% Zn-10 wt% Cu alloy at 400 °C, as represented in the portion of the Cu-Zn phase diagram shown below (at point A).
Examine the following phase diagram and identify the ... Examine the following phase diagram and identify the feature represented by point B. A B 760 torr Temperature. A ) melting point. D ) sublimation point. B ) triple point. E ) boiling point. C ) critical point. 16 . Consider the following phase diagram and identify the process occurring as one goes from point C to point D.

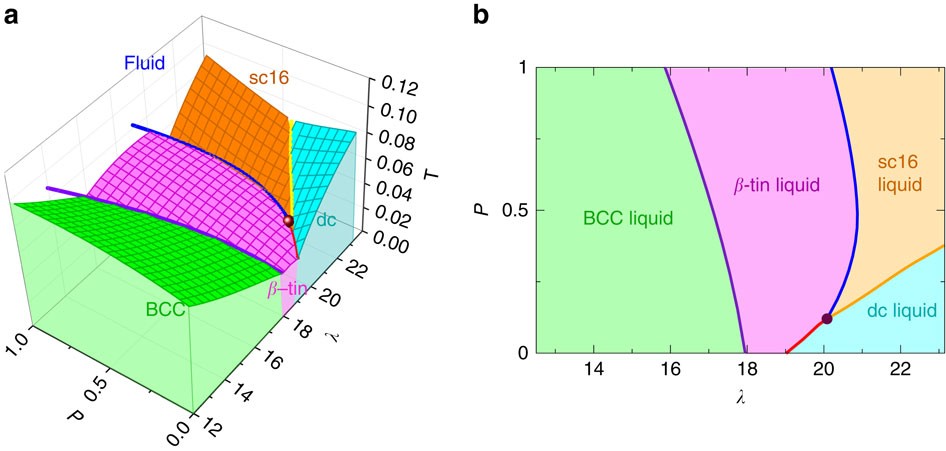

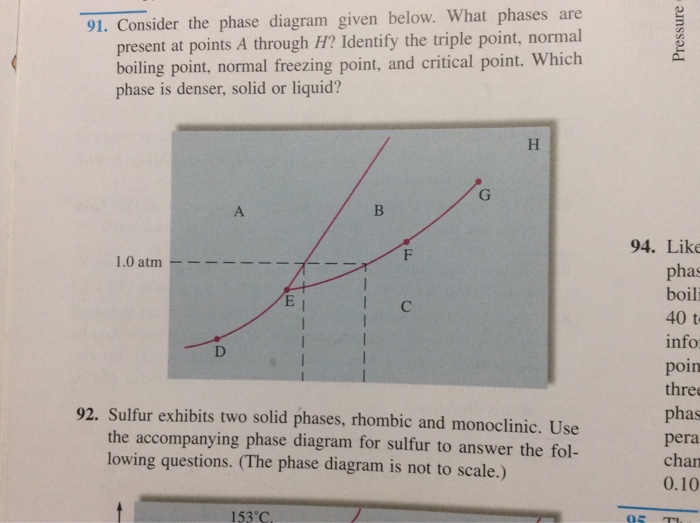


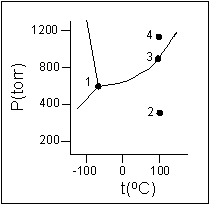



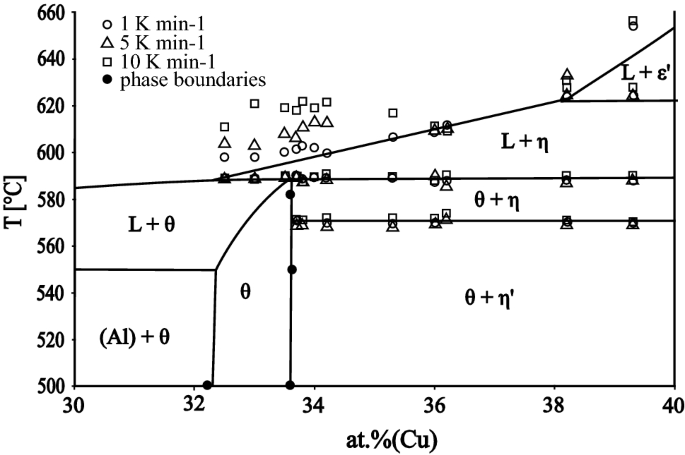











0 Response to "43 examine the following phase diagram and determine what phase exists at point d."
Post a Comment