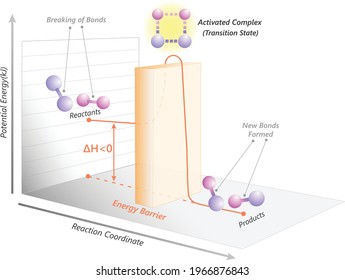
44 potential energy diagram exothermic
PDF Exothermic vs endothermic reaction graphs State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer-- PDF Potential Energy Diagram Worksheet ANSWERS The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.

Potential energy diagram exothermic
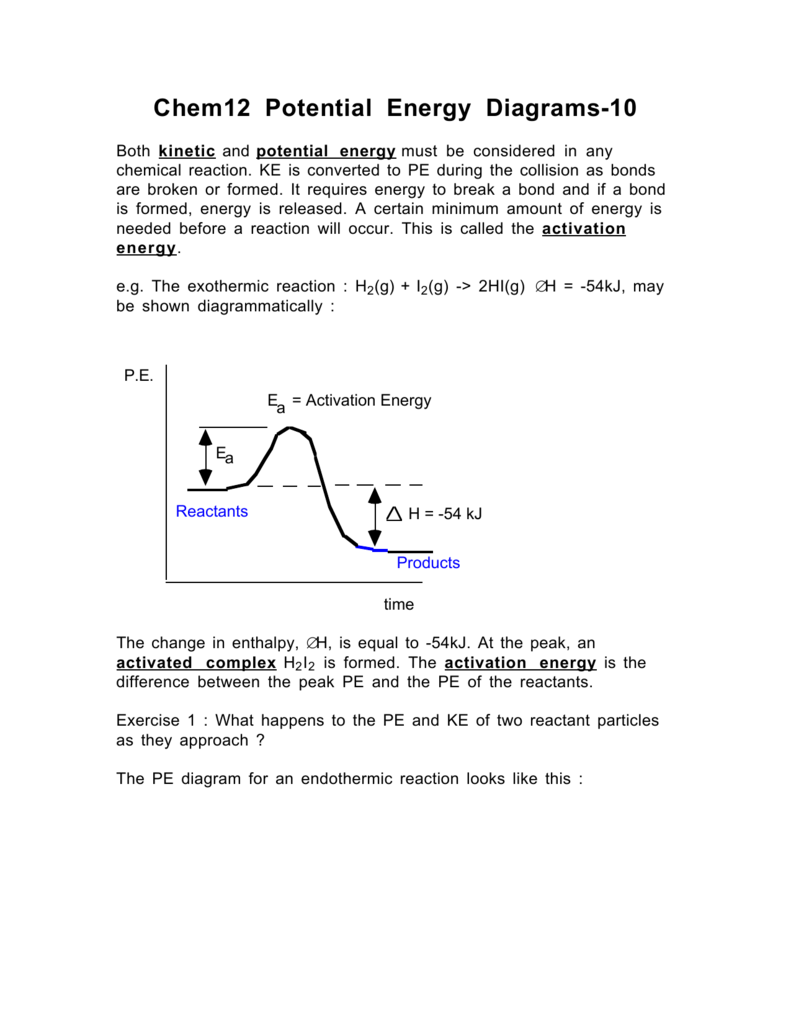
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... Decrease in kinetic energy - energy released - exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. The reactants and products Potential Energy Diagram Worksheet - Worksheet Smart Potential energy diagram worksheet 1. Draw a line segment that represents the potential energy of the reactants and label the line. Draw an energy diagram for a reaction. Nat is tne cnmatpy change ah tor the d what is the enthalpv ch forward reaction. Is the overall reaction as shown exothermic or endothermic. 14 Sketch a potential energy diagram for an exothermic ... 14. Sketch a potential energy diagram for an exothermic reaction and for an endothermic reaction. Label the axes, reactants, products, heat of reaction, activation energy, and transition state on each diagram. 15. The following data were obtained for the decomposition of dinitrogen pentoxide at 45 C. N 2 O 5 (mol/L) Time (min) 0.316 0 0.274 39 0.238 80 0.190 140 0.146 210 a) Plot a graph of ...
Potential energy diagram exothermic. Reaction Energy Diagrams CHM 233 Review Arizona State University in Tempe, AZ is a public research university ranked #1 in the U.S. for innovation, dedicated to accessibility and academic excellence. 18.3 Potential Energy Diagrams - CK-12 February 23, 2012 - ABOUT · Our Mission · Meet the Team · Partners · Press · Careers · Security · Status · Success Stories · Overview EXOTHERMIC & ENDOTHERMIC REACTIONS: ENERGY DIAGRAMS ENERGY DIAGRAM. • A = POTENTIAL ENERGY OF REACTANTS. • B = ACTIVATION ENERGY. • ENERGY REQUIRED TO START THE REACTION. • C = ACTIVATION ENERGY (REVERSE. How to Read Potential Energy Diagrams May 2, 2017 - A small activation energy reaction ... Very exothermic reaction with a small activation energy. Energy level diagram for an endothermic chemical reaction without showing the activation energy. Moderately endothermic reaction with a moderately high activation energy. POTENTIAL ENERGY ...
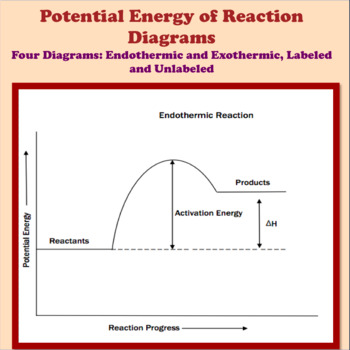
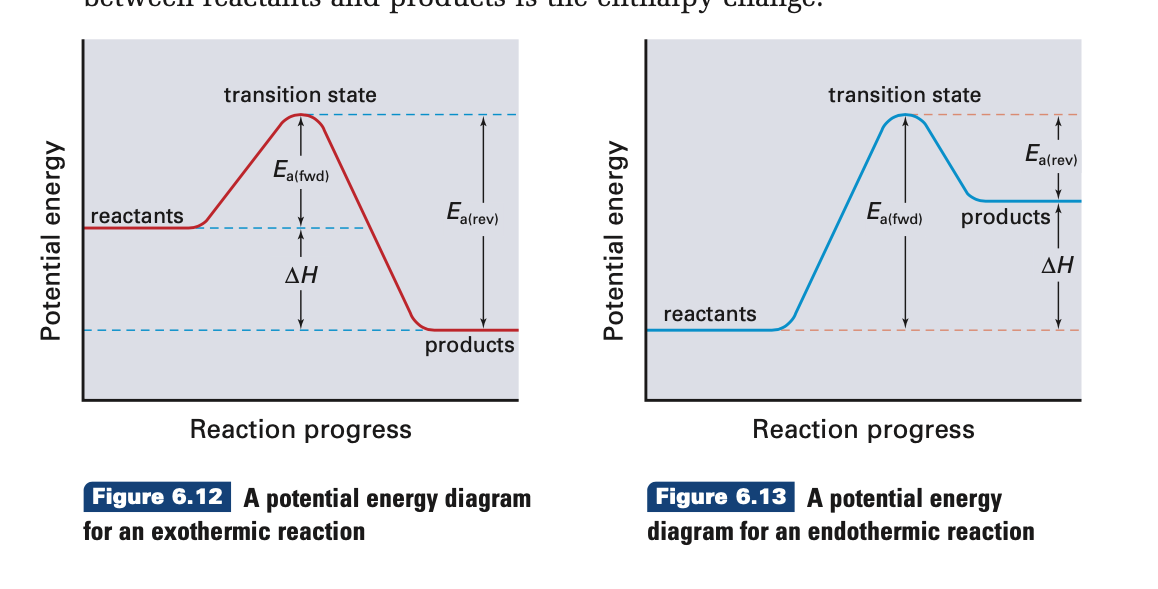
Potential Energy Diagrams | CK-12 Foundation ABOUT · Our Mission · Meet the Team · Partners · Press · Careers · Security · Status · Success Stories · Overview Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited ... Oct 7, 2015 - This Pin was discovered by Eric Tobias. Discover (and save!) your own Pins on Pinterest 18.4: Potential Energy Diagrams - Chemistry LibreTexts 1 month ago - The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy ... Solved Which potential energy diagram represents an ... Question: Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential ...
6.6: Potential Energy Diagrams - Chemistry LibreTexts A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for ... Potential Energy Diagram: study guides and answers on Quizlet Potential Energy Diagram Chemical Energy Enthalpy Of Formation Energy Specific Heat Capacity. TERMS IN THIS SET (25) Which describes the enthalpy change associated with an endothermic reaction? It is positive because the enthalpy of the products is greater than the enthalpy of the reactants. Endothermic vs. exothermic reactions (article) | Khan Academy If you're seeing this message, it means we're having trouble loading external resources on our website · If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked How to draw the potential energy diagram for this reaction ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
Dublin Schools - Lesson : Exothermic and Endothermic The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams.
Draw the potential energy diagram for an exothermic ... Draw the potential energy diagram for an exothermic reaction. Explain the terms: activation energy of backward reaction. Updated On: 21-7-2021. This browser does not support the video element. Answer. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
How do you know if a potential energy diagram is ... Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams. The total potential energy of the system increases for the endothermic reaction as the system absorbs energy from the surroundings. What is the order of E1 elimination reaction?
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
Potential Energy Diagram Worksheet Answers - Thekidsworksheet Some of the worksheets for this concept are chemistry 12 work 1 2 potential energy diagram work answers potential energy diagram work conservation of energy work name name period date chemistry energy work answer key physics work and energy bar graph work potential energy total. Is the reaction in 6 exothermic or endothermic.
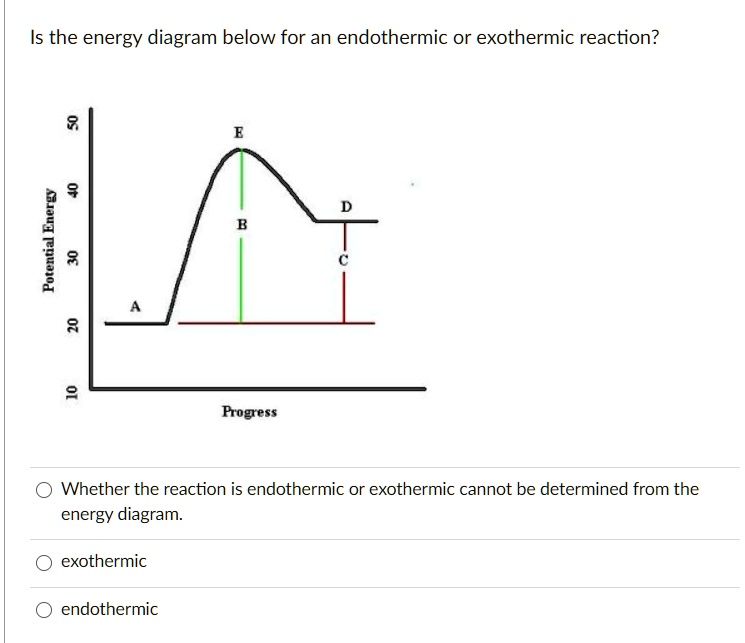
(Get Answer) - Based on the following energy diagram, is ... H3.17 The potential energy diagram below represents a reaction. Which arrow represents the activation energy of the forward reaction? 3 2 Reaction coordinate OA O B H3.21 which potential energy diagram represents an exothermic reaction? Reaction...
Solved Select the potential energy diagram that represents ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Exothermic reactions are characterized by negative change in …. View the full answer. Transcribed image text: Select the potential energy diagram that represents an exothermic (exergonic) reaction.
What is a potential energy diagram? - Moorejustinmusic What is a potential energy diagram? A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Potential energy diagrams for endothermic and exothermic reactions are described. How do you define kinetic and potential energy?
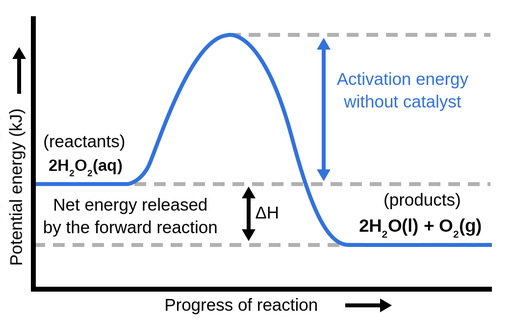
How can I represent an exothermic reaction in a potential energy ... December 19, 2014 - An exothermic reaction is a reaction in which energy is given off, or, in other words, a reaction that has a ... #6CO_2 + 6H_2O#); this difference in potential energy (on this diagram labeled as the energy released by the forward raection or required for the reverse reaction ) represents
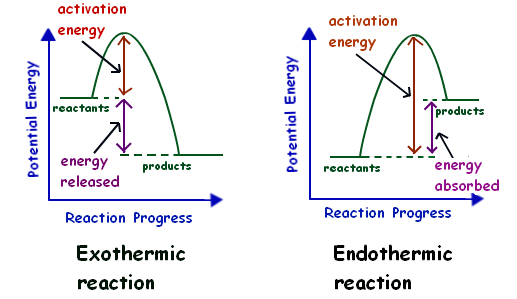
Where is the activation energy of an exothermic graph ... The activation energy for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products. What is the activation energy of an exothermic reaction? Figure 12.4: The energy changes that take place during an exothermic reaction.
Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
Potential Energy Diagram Worksheet Key - Forest Hills High ... March 16, 2017 - Good Day Forest Hills.... January 2022 Winter Edition · It is the policy of the New York City Department of Education to provide equal educational opportunities without regard to actual or perceived race, color, religion, creed, ethnicity, national origin, alienage, citizenship status, disability, ...
Exothermic and Endothermic Processes | Introduction to Chemistry Endothermic and exothermic reactionsPaul Andersen explains how heat can be absorbed in endothermic or released in exothermic reactions. An energy diagram can be used to show energy movements in these reactions and temperature can be used to measure them macroscopically.
18.4: Potential Energy Diagrams - Chemistry LibreTexts Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
Classify each chemical reaction as exothermic or ... Potential energy diagram for the reaction Potential energy diagram for the reaction 24,0 + 572 kJ → 2H2 + O2 H₂.0₂ Potential Energy H,O Time o exothermic O endothermic DONE 2 See answers Advertisement Advertisement karlaperez217 karlaperez217 Answer: Endothermic . next one is exothermic.
Learn Potential Energy Diagram in 3 minutes. - Toppr Get a quick overview of Potential Energy Diagram from Effect of Temperature on Rate of Reactions ... This type of reaction is known as Exothermic reaction ...
Amazing Exothermic Energy Diagram Life Processes Class 10 ... Exothermic energy diagram. U Ufinal Uinitial 1. It is quite possible to tell if the reaction is exothermic or not. Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction this is why products have less energy Δ H will be negative. More rigorous Gibbs free energy spontaneity relationship.
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
PDF Potential Energy Diagrams potential energy diagrams are a visual representation of potential energy in a chemical reaction note the x axis is usually reaction cordinate or time ... exothermic (loss of energy) if reactants have less energy then products, the reaction is endothermic (gain of energy)
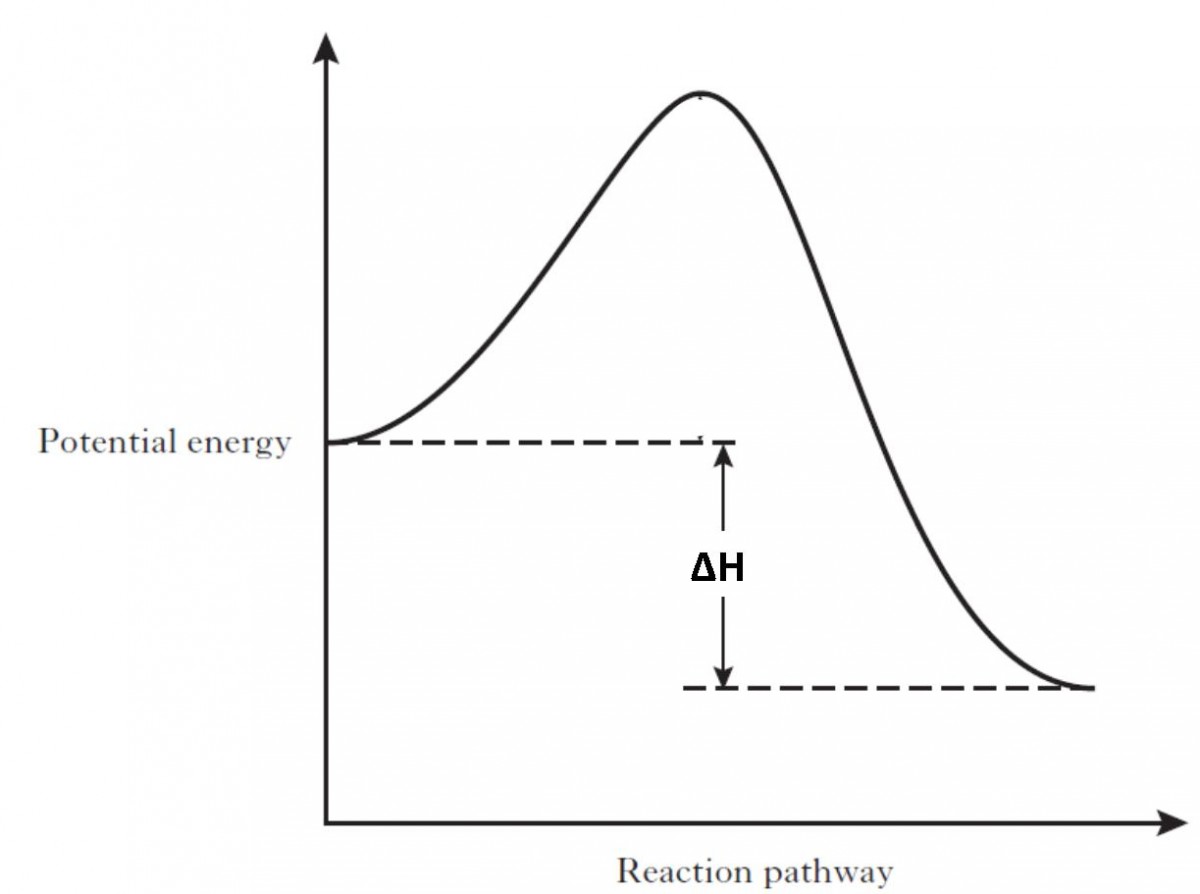
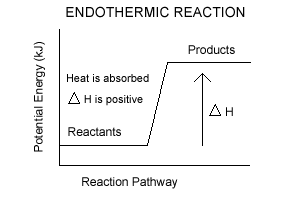
Chemistry Journal_Module 7 Energy in Reactions-1.docx ... In an exothermic reaction , the products have less potential energy than the reactants had . This is why the potential energy diagram for an exothermic reaction starts at a higher energy value and ends at a lower energy value . In an endothermic reaction , the products end up with more stored potential energy than the reactants .
Representing endothermic and exothermic processes using ... Representing endothermic and exothermic processes using energy diagrams AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
How can I represent an endothermic reaction in a potential energy ... Why is the respiration reaction exothermic? Why is combustion an exothermic reaction? How can I read the potential energy diagrams when there is ...
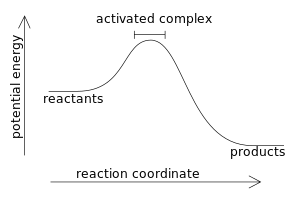
PDF NAME Kinetics Potential Energy Diagrams - ISD 622 Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants), through stage 2 (activated complex to), to stage 3 (products ...
Potential Energy Diagrams 2016.notebook March 4, 2016 - 6:30 PM - 8:30 PM Elementary Schools Parent/Teacher Conferences · 7:30 PM Joint Middle School & High School PTA Meeting at the High School
Energy Diagram — Overview & Parts - Expii Exothermic reactions uses/loses energy/heat during the reaction, so when drawing the energy diagram, you want the reactants on the graph to be higher than the ...
Welcome to CK-12 Foundation | CK-12 Foundation Look at the two exothermic reactions whose potential energy diagrams are represented in figures A and B below, and notice the activation energy marked in each. When a reaction has a low activation energy, like in figure A above, most of the reactant molecules have sufficient kinetic energy to react, and the reaction will most likely be rapid (a ...
ws_potential_energy_diagram_answers.pdf - Potential Energy ... Complete the potential energy diagram for an exothermic reaction, where the reactants have 250 kJ of potential energy stored in their chemical bonds, there is 100 kJ of activation energy required and the products have 50 kJ of potential energy stored in their chemical bonds. Scale and label the y-axis for kJ of potential energy.
Potential energy diagrams - Controlling the rate - Higher ... Potential energy diagrams can be used to calculate both the enthalpy change and the activation energy for a reaction. An exothermic reaction is one in which heat energy is given out. The products must have less energy than the reactants because energy has been released.
Potential Energy Diagrams | CK-12 Foundation 9 Jul 2019 — Figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change ...
How does the energy level diagram show this reaction is ... Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
14 Sketch a potential energy diagram for an exothermic ... 14. Sketch a potential energy diagram for an exothermic reaction and for an endothermic reaction. Label the axes, reactants, products, heat of reaction, activation energy, and transition state on each diagram. 15. The following data were obtained for the decomposition of dinitrogen pentoxide at 45 C. N 2 O 5 (mol/L) Time (min) 0.316 0 0.274 39 0.238 80 0.190 140 0.146 210 a) Plot a graph of ...
Potential Energy Diagram Worksheet - Worksheet Smart Potential energy diagram worksheet 1. Draw a line segment that represents the potential energy of the reactants and label the line. Draw an energy diagram for a reaction. Nat is tne cnmatpy change ah tor the d what is the enthalpv ch forward reaction. Is the overall reaction as shown exothermic or endothermic.
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... Decrease in kinetic energy - energy released - exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. The reactants and products































0 Response to "44 potential energy diagram exothermic"
Post a Comment