41 molecular orbital diagram b2
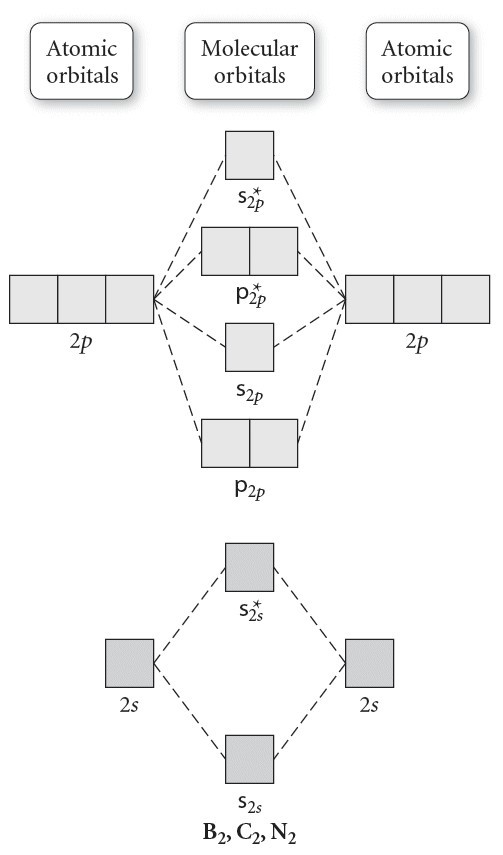
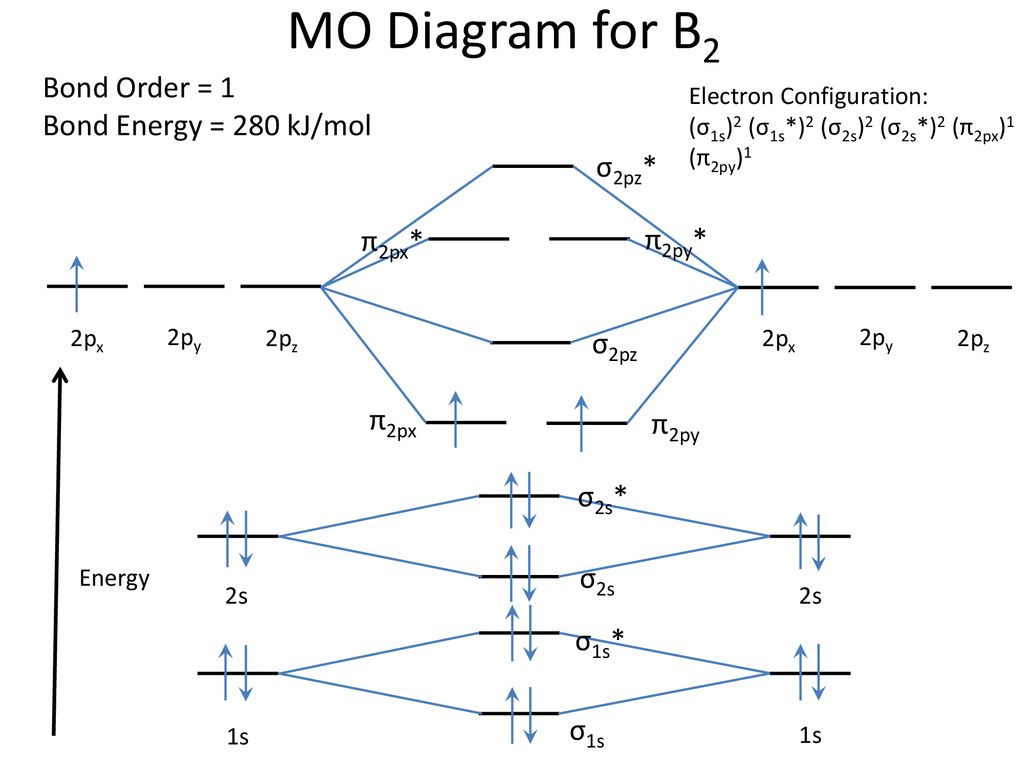
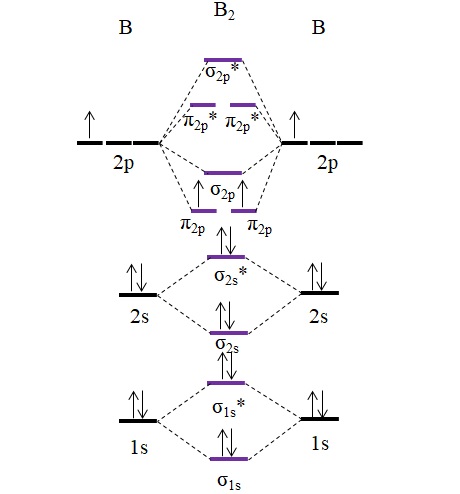
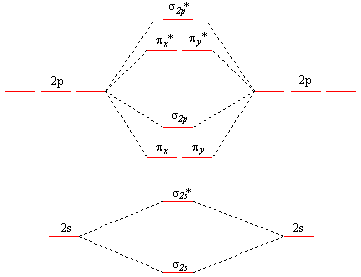
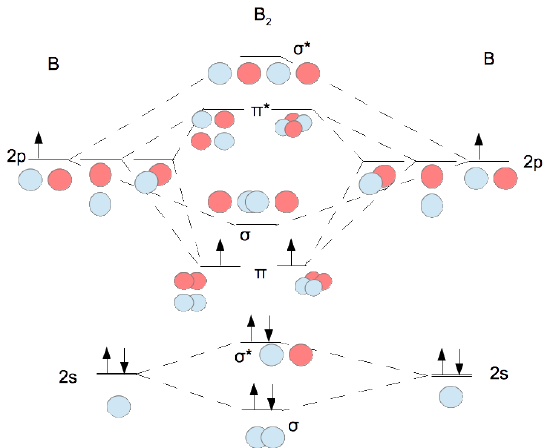
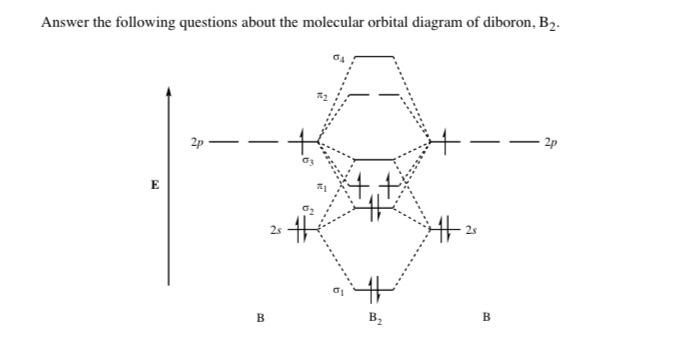
7.7 Molecular Orbital Theory - Chemistry Fundamentals The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. M.O. Diagram for B2 - CHEMISTRY COMMUNITY As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond.
Molecular Orbital Diagram Be2 - schematron.org + and Be2.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. 1.

Molecular orbital diagram b2
MO Diagrams - GitHub Pages That's it for the MO diagram of `B_2`! To check, count how many electrons there are in total. `B_2` has `2(3)=6` valence electrons. The MO diagram has `6` electrons as well. Notice that the last two electrons go into two separate `pi` orbitals instead of filling 2 electrons into one orbital. This is in accordance to Hund's Rule. #2. Inorganic Chemistry | Vol 61, No 9 Mar 07, 2022 · Actinyl ions (AnO22+), with their linear geometry, chemical stability, and prevalence in a wide range of solid and solution state forms, exemplify the distinctive electronic structure of the actinide elements. Spectroscopic measurements of field gradient tensors and magnetic parameters in crystalline samples of Cs2UO2Br4 (left) and Cs2UO2Cl4 (right) in conjunction with ab initio calculations ... 36 bh2 molecular orbital diagram - Diagram For You 36 bh2 molecular orbital diagram. 4 days ago — Walsh correlation diagram is a plot of molecular orbital energy as a function of some systematic change in molecular geometry. The wave functions, level energies and Mülliken population analysis of localized molecular orbitals (LMO's) for B4Cl4, 1,5-C2B3H5 and the closo-BnH2-n (n = 6-10, 12) are ...
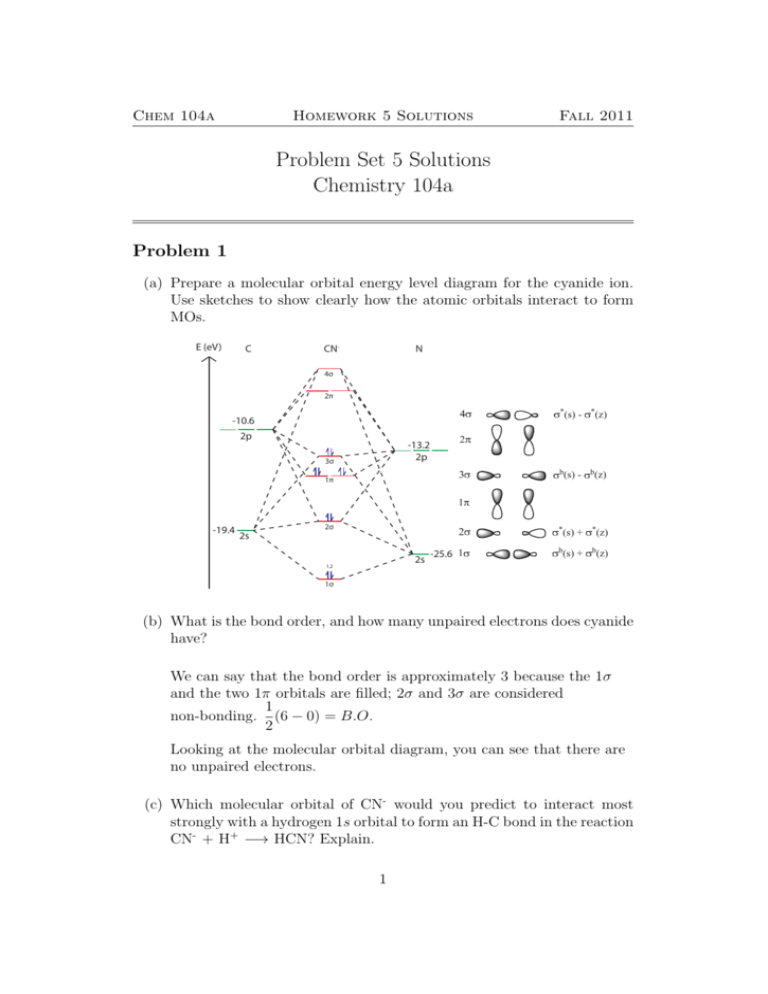
Molecular orbital diagram b2. CN- lewis structure, molecular orbital diagram, and, bond order Procedure to draw the molecular orbital diagram of CN. 1. Find the valence electron of each atom in the CN molecule. Clearly, carbon has 4 valence electrons and nitrogen has 5. 2. Find if the molecule homo-nuclear diatomic molecular orbital or hetero-nuclear diatomic molecular orbital. Clearly, CN is hetero orbital. 3. Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages 2) Use molecular orbital diagrams to determine which of the following are paramagnetic. A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2 B2 Molecular Orbital Diagram - Summarized by Plex.page ... The final valence molecular orbital diagram is shown below. Though basic models are sufficiently detailed in Valence Bond Theory and Lewis Structures, the Molecular Orbital Theory provides answers to more complicated questions. In the table below, a list of atomic orbital orbitals of 2 Hydrogen atoms orbiting the axis of the bond is outlined. Draw MOT diagram for B2 molecule and calculate its class ... The molecular orbitals having the same sign combine and give bonding molecular orbitals. We have to draw the molecular orbital diagram for B 2 molecule. The B 2 molecule is formed by the combination of two boron atoms. The two boron atoms are linked by a covalent bond. The atomic number of boron is 5.
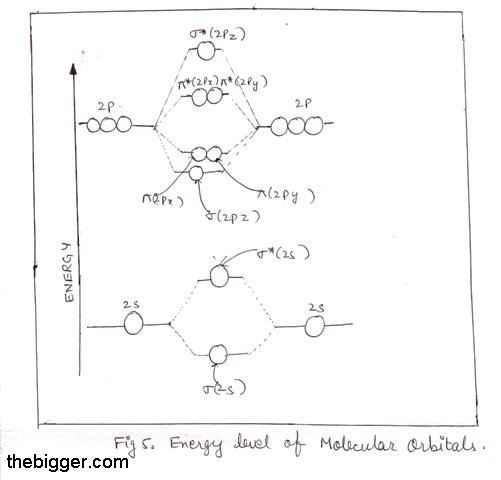
Molecular Orbital Theory - Texas A&M University Draw the molecular orbital diagram for B 2. The number of unpaired electrons in the B 2 molecule is _____. (a) zero (b) 1 (c) 2 (d) 3 (e) 4 8. Which one of the following statements is false? (a) Valence bond theory and molecular orbital theory can be described as two different views of the same thing. Molecular Orbital Diagram of B2, C2, and N2 Molecules ... From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ... (PDF) general-chemistry.pdf | Sumit Banerjee - Academia.edu Academia.edu is a platform for academics to share research papers. PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
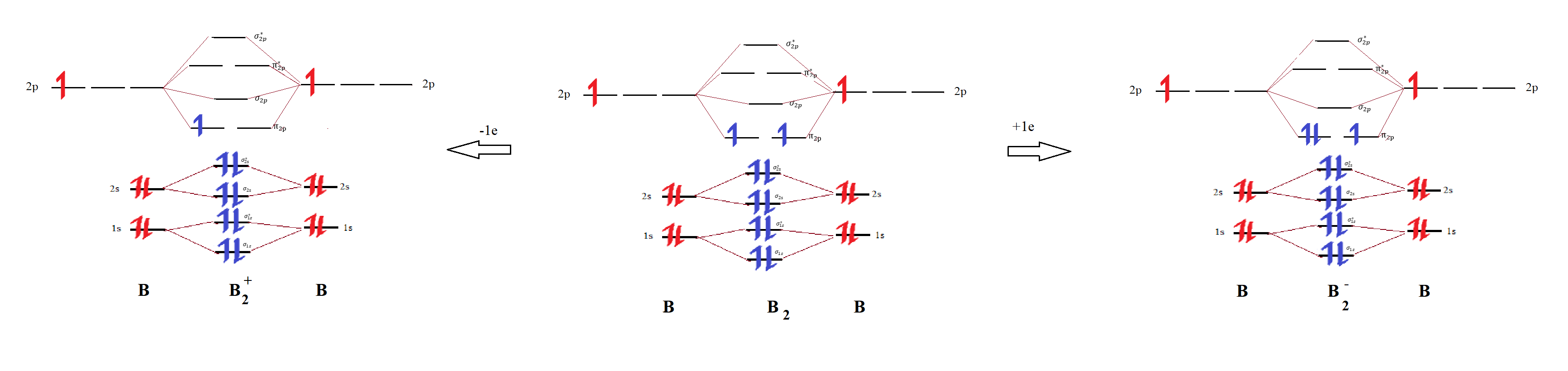
Answered: What is the bond order in LiF? A. 0 B.… | bartleby The following molecular orbital diagram belongs to a molecule of the type XH2 (where X is an unidentified 2nd row element). Do you think the molecule is bent or linear? Explain your reasoning. Make approximate sketches of the bonding and non-bonding molecular orbitals. Solved Draw the molecular orbital diagram for B2+ (this is ... Science Chemistry Chemistry questions and answers Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero Why is there a difference between O2 and B2 sigma 2p ... The molecular orbital diagram for $\\ce{O2}$ says that the sigma 2p bonding molecular orbital is lower in energy than the pi 2p bonding molecular orbital. Why is this not the case in the $\\ce{B2}$ MO What Is The Bond Order Of B2 - questionfun.com 1 So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. Nov 11, 2016
Be2 Molecular Orbital Diagram - schematron.org Nov 11, · As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond.
Solved Part A By drawing molecular orbital diagrams for B2 ... Part A By drawing molecular orbital diagrams for B2, C2, N, O,, and F2. predict which of these homonuclear diatomic molecules are magnetic. View Available Hint (s) F2 02 and B2 02 and F2 02 Submit Part B Based on the molecular orbital diagram for NO, which of the following electronic configurations and statements are most correct?
molecular orbital diagram of B2 - Brainly.in Molecular orbital diagram of B2 1 See answer manfoosah2002 is waiting for your help. Add your answer and earn points. priyesharma02 priyesharma02 Molecular orbita diagram of B2 New questions in Chemistry. ejj-bgir-qdr hajqjqbwvgwhw short note on all the types of Quantum numbers.
Molecular Orbital Theory. B2 - YouTube This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the MO diagrams for B2.
Molecular Orbital Theory - Purdue University into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below. Experiments have shown that O2and F2are best described by the model in the figure above, but B2, C2, and N2are best described by a model that includes hybridization, as shown
Answered: How of the following molecules are… | bartleby (Use the molecular orbital diagram) many B2, C2, N2, O2 A. 0 В. I С. 2 D. 3 E. 4. Question. 29. Transcribed Image Text: E. Molecular orbitals alv ( 29. How many of the following molecules are paramagnetic? (Use the molecular orbital diagram) B:, C2, N2, O2 A. 0 В. 1 C. 2 D. 3 E. 4 Energy A 30. Use the molecular orbital diagram to figure out ...
Draw the molecular orbital diagram for:(i) Be2(ii) B2 and ... (i) Be2 molecule: The electronic configuration of Be(Z = 4) is: 4 Be 1s 2 2s 1 Be 2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. Number of valence electrons in Be atom = 2 Thus in the formation of Be 2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies.
Chapter 9 Molecular Orbitals in Chemical Bonding (Midterm ... molecular orbital diagram for F2. number of elections in the sigma*2p molecular orbital is 0 the total numbers of electrons in the pi2p molecular orbital of B2 is
Institute Of Infectious Disease and Molecular Medicine For information on South Africa's response to COVID-19 please visit the COVID-19 Corona Virus South African Resource Portal.
What is the molecular orbital diagram for B_2? | Socratic Jan 27, 2015 Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals.
C22- Molecular Orbital Diagram B2 MO diagram with no sp mixing: B2 = 6 e⁻. Label the sigma bonding molecular orbitals on the diagram above using the designation. d. Give the electron configurations for the species C2 and C When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz.
What is the molecular orbital diagram for B2? - Clutch Prep Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape . There are two types of MO diagrams: Recall that the bonding MOs are those without an asterisk (e.g., σ1s), while the antibonding MOs are those with an asterisk (e.g., σ1s*).
(PDF) Engineering Chemistry by Jain & Jain | Inder Rahi ... Academia.edu is a platform for academics to share research papers.
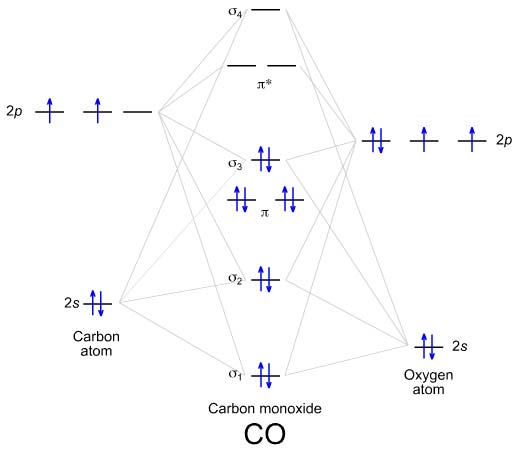
PDF Miessler-Fischer-Tarr5e SM Ch 05 CM molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p * orbitals exhibit Cs symmetry. The latter do not possess C2 rotation axes coincident to the
Beta decay - Wikipedia Description. The two types of beta decay are known as beta minus and beta plus.In beta minus (β −) decay, a neutron is converted to a proton, and the process creates an electron and an electron antineutrino; while in beta plus (β +) decay, a proton is converted to a neutron and the process creates a positron and an electron neutrino.
Molecular Orbital Diagram Be2 Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be Indicate theirnumbers of unpaired electron and mention. The first ten molecular orbitals may be arranged in order of energy as follow: σ (1s ) ∗ (1s) Molecular orbital energy level for Be2.
39 b2+ molecular orbital diagram - Diagram For You B2+ molecular orbital diagram. Beta decay - Wikipedia Molecular band spectra showed that the nuclear spin of nitrogen-14 is 1 (i.e., equal to the reduced Planck constant) and more generally that the spin is integral for nuclei of even mass number and half-integral for nuclei of odd mass number.
36 bh2 molecular orbital diagram - Diagram For You 36 bh2 molecular orbital diagram. 4 days ago — Walsh correlation diagram is a plot of molecular orbital energy as a function of some systematic change in molecular geometry. The wave functions, level energies and Mülliken population analysis of localized molecular orbitals (LMO's) for B4Cl4, 1,5-C2B3H5 and the closo-BnH2-n (n = 6-10, 12) are ...
Inorganic Chemistry | Vol 61, No 9 Mar 07, 2022 · Actinyl ions (AnO22+), with their linear geometry, chemical stability, and prevalence in a wide range of solid and solution state forms, exemplify the distinctive electronic structure of the actinide elements. Spectroscopic measurements of field gradient tensors and magnetic parameters in crystalline samples of Cs2UO2Br4 (left) and Cs2UO2Cl4 (right) in conjunction with ab initio calculations ...
MO Diagrams - GitHub Pages That's it for the MO diagram of `B_2`! To check, count how many electrons there are in total. `B_2` has `2(3)=6` valence electrons. The MO diagram has `6` electrons as well. Notice that the last two electrons go into two separate `pi` orbitals instead of filling 2 electrons into one orbital. This is in accordance to Hund's Rule. #2.



















0 Response to "41 molecular orbital diagram b2"
Post a Comment