43 endothermic reaction coordinate diagram
Draw an energy diagram for an endothermic reaction without ... Explanation: Endothermic reactions are defined as the reactions in which energy of the product is greater than the energy of the reactants.The total energy is absorbed in the form of heat and for the reaction comes out to be positive.. Catalyst is the substance which does not participate in the reaction but increases the rate of the reaction and results in the early formation of product. PDF Exothermic Endothermic "downhill" "uphill" 7. Be able to recognize and read potential energy diagrams. Reaction Coordinate Reaction Coordinate Exothermic Endothermic "downhill" "uphill" 8. ∆H is (+) for endothermic reactions and is (-) for exothermic reactions. 9. The rates of the forward and reverse reactions are equal at equilibrium. 10.
An endothermic reaction with high activat... - Physical ... An endothermic reaction with high activation energy for the forward reaction is given by the diagram : 4 Potential energy Potential energy (1) (2) Reaction coordinate Reaction coordinate Potential energy Potential energy (3) (4) Reaction coordinate Reaction coordinate < > Answer

Endothermic reaction coordinate diagram
SOLVED:Draw reaction coordinate diagram for slow ... Draw reaction coordinate diagram for slow, concerted endothermic reaction: Label axes_ reactant, product, transition state, activation energy and AG. Draw reaction coordinate diagram for slow stepwise reaction that favors reactants. An endothermic reaction with high activation energy for ... In an endothermic reaction, the overall energy is absorbed or taken in by the system and therefore,the energy of the product is higher than that of the reactant. An endotheremic reaction with high activation energy for the forward reaction is given by the diagram C. Analyzing Energy With a Reaction Coordinate Diagram ... And a reaction coordinate diagram where the energy level of B ends up lower than A is exothermic (delta E is negative): Activation Energy The activation energy is important in a reaction. Even if...
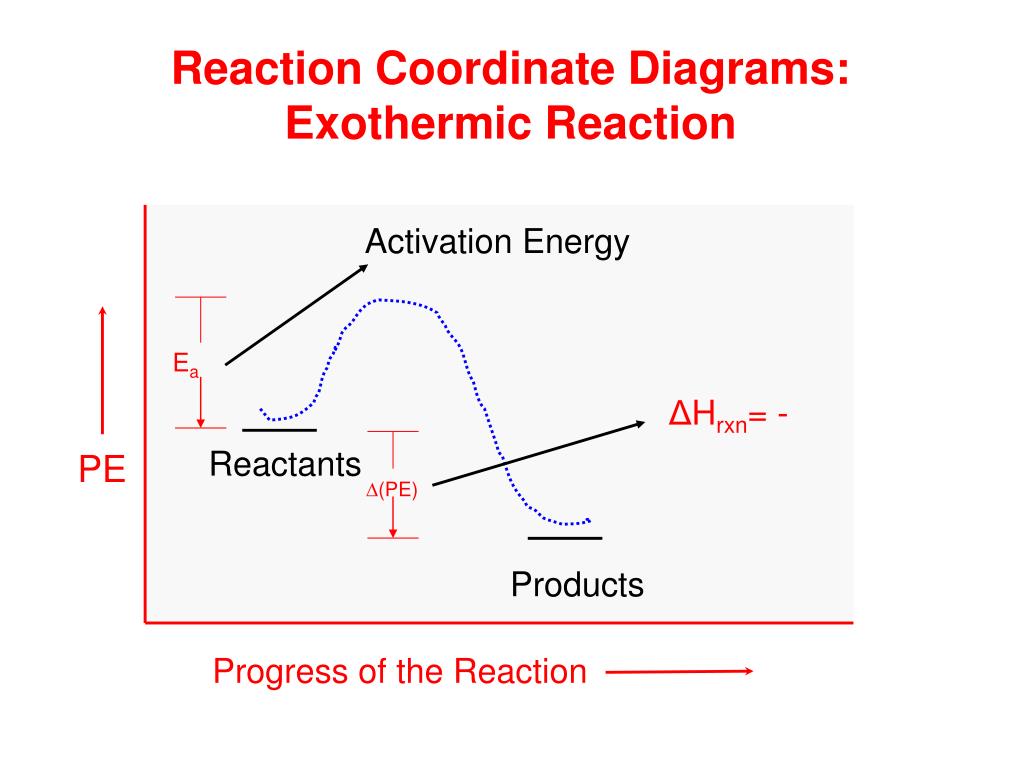
Endothermic reaction coordinate diagram. Reaction Coordinate Diagrams - University of Illinois Urbana ... The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction (heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram. Energy profile (chemistry) - Wikipedia Reaction coordinate diagrams. The intrinsic reaction coordinate (IRC), derived from the potential energy surface, is a parametric curve that connects two energy minima in the direction that traverses the minimum energy barrier (or shallowest ascent) passing through one or more saddle point(s). However, in reality if reacting species attains enough energy it may deviate from the IRC to some extent. Solved Which reaction coordinate diagram shows an | Chegg.com Which reaction coordinate diagram shows an endothermic reaction with no intermediates? free energy. kJ/mol mah reaction coordinate A. reaction coordinate B. reaction coordinate C. reaction coordinate D. ОА OB Ос OD O None of them Which of the following can undergo keto-enol tautomerization? он ОН он ОН І ІІ III IV ОТ OIL ОІІІ O IV O All of these Arrhenius Theory and Reaction Coordinates Typically, we envision reactions proceeding left to right along the reaction coordinate, so often, the activation energy is only noted for the forward reaction. The activation energy on the diagram below shows the barrier to be 102.6 kJ mol -1 .
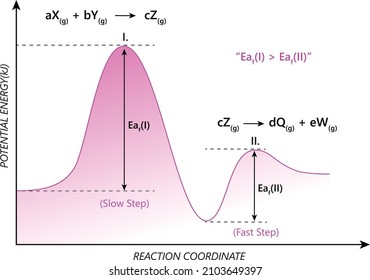
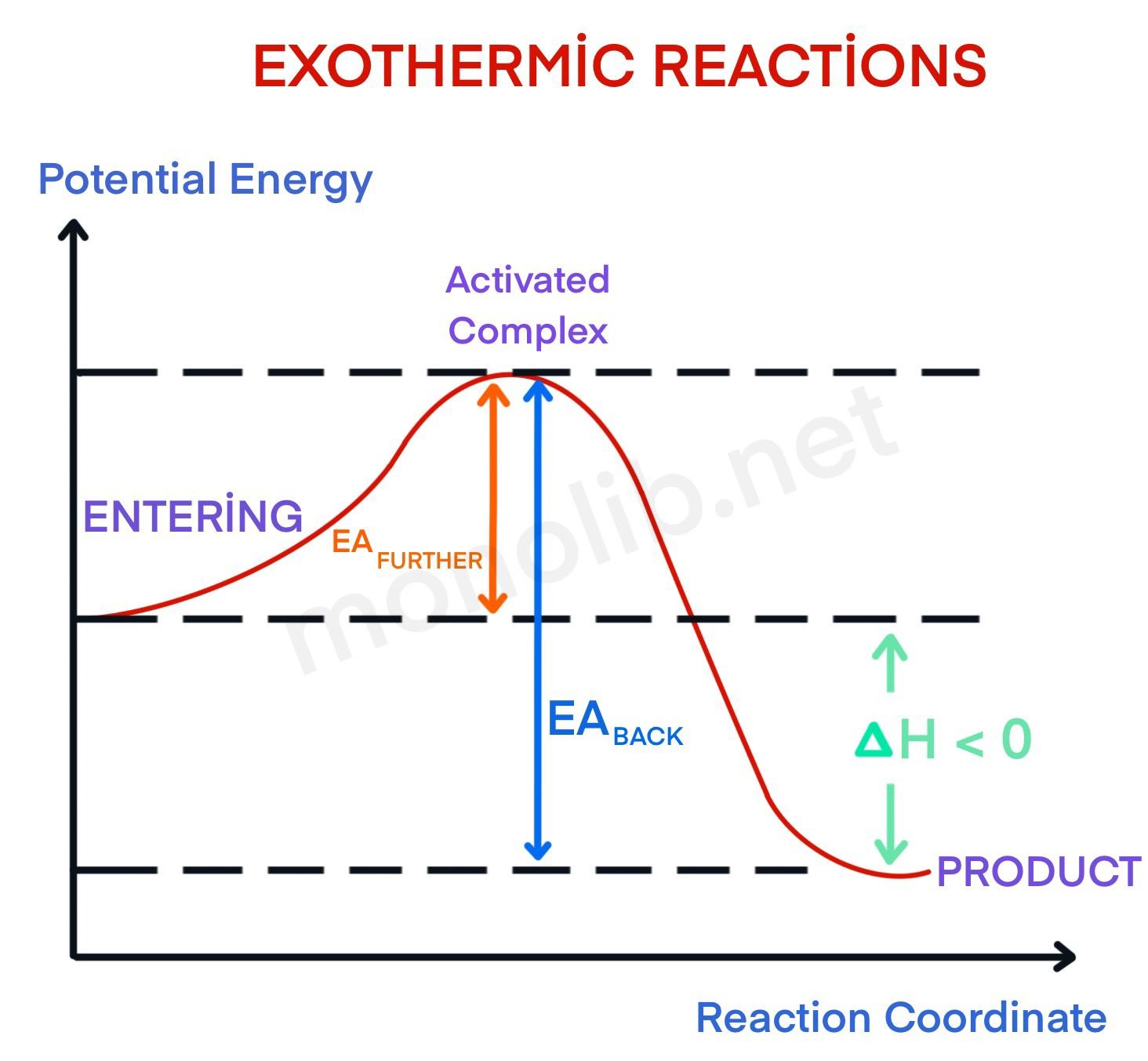
What are Endothermic Reactions? (with Examples & Video) The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant. Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Reaction Coordinate Diagram Endothermic Vs Exothermic Oct 01, 2019 · A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction! 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic. 6. Reaction Coordinate Diagram - VIZISCIENCE® INTERACTIVE LABS The reaction coordinate diagram is represented below: Things to note ΔH represents the difference between enthalpy of reactants and products. Ea1 and Ea2 represent the activation energy for step 1 and step 2 in the reaction. The step with the highest activation energy is the slowest step reaction.
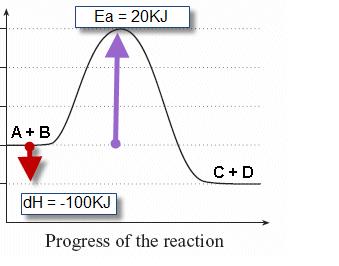
Chem 233 Exam 3 Flashcards - Quizlet In an exothermic reaction the transition state resembles the _____, whereas in an endothermic reaction the transition state more closely resembles the _____ reactants, products. Select all the statements that correctly describe the reaction coordinate vs. energy diagram for an SN1 reaction. A. The energy diagram shows two energy maxima ... PDF Energy/Reaction Coordinate Diagrams 1! Energy/Reaction Coordinate! Diagrams! Thermodynamics, Kinetics ! Dr. Ron Rusay" A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change (ΔGo) Enthalphy (ΔHo): the heat given off or absorbed during a reaction PDF Thermodynamics vs Kinetics - Columbia University A general Reaction Coordinate Diagram relating the energy of a system to its geometry along one possible reaction pathway is given in the figure below. In the figure below, the Activation Energy, Ea is that critical minimum energy in a chemical reaction required by reactants to be converted into products. the quantities, Ea; Answered: 1. a) Write the reaction profile… | bartleby Transcribed Image Text: 1. a) Write the reaction profile (reaction coordinate diagram) for an endothermic reaction that occurs via a 3 step mechanism with the second step being the rate determining step? b) Label reactants and products, each activation energy and the enthalpy change for the reaction. c) Label the location of intermediates.
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. This means that there was more energy in reactants than in the products.
PDF Chapter 5. Reactions of Alkenes and Alkynes Learning ... Chapter 5. Reactions of Alkenes and Alkynes Learning objectives: 1. Identify the followings from a reaction coordinate diagram when applicable: endothermic or exothermic reactions, activation energy, heat of reaction, locations of transition states, locations of intermediates, and rate-limiting step. 2.
What is a reaction coordinate diagram? - FindAnyAnswer.com An energy diagram can be defined as a diagram showing the relative potential energies of reactants, transition states, and products as a reaction progresses with time. One can calculate the E a c t E_ {act} Eact?E, start subscript, a, c, t, end subscript and Δ H ΔH ΔH for any reaction from its energy diagram.
Endothermic Reaction Coordinate Diagram - schematron.org Sep 16, 2018 · The fully filled in reaction coordinate diagram is displayed below. This reaction is also exothermic because the energy of the products is lower than that of the. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other.Start studying CHEMISTRY 2.
6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this:
Endothermic Reaction Coordinate Diagram - Wiring Diagrams A typical reaction coordinate diagram for a mechanism with a single step is shown below: Below is a reaction coordinate diagram for an endothermic reaction. In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other.
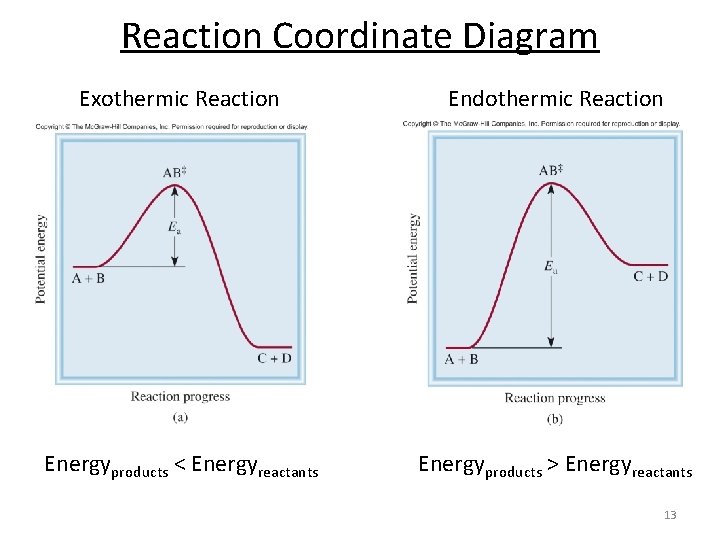
Reaction Coordinate Diagram Endothermic - Wiring Diagrams The " reaction coordinate " plotted along the abscissa represents the diagrams can describe both exothermic and endothermic reactions.A reaction will be exothermic if the energy of the products is less than the energy of the reactants. A reaction is endothermic when the energy of the products is greater than the energy of the reactants.
Solved 1. Draw reaction diagram (or reaction coordinate ... 1. Draw reaction diagram (or reaction coordinate, reaction progress, etc) of an endothermic reaction. Clearly label Reactant, Product, Transition State, AHss, Activiation Energy (Ea) 2. Connect the lines to match the impact (note how big an impact on equilibrium or reaction rate by small changes in Aton or activation energy!!)
04.02 Reaction Coordinate Diagrams and Stability Trends ... General structure of a reaction coordinate diagram, including transition states and intermediates. Overall free energy change and activation energy. Definiti...
Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants.
Analyzing Energy With a Reaction Coordinate Diagram ... And a reaction coordinate diagram where the energy level of B ends up lower than A is exothermic (delta E is negative): Activation Energy The activation energy is important in a reaction. Even if...
An endothermic reaction with high activation energy for ... In an endothermic reaction, the overall energy is absorbed or taken in by the system and therefore,the energy of the product is higher than that of the reactant. An endotheremic reaction with high activation energy for the forward reaction is given by the diagram C.
SOLVED:Draw reaction coordinate diagram for slow ... Draw reaction coordinate diagram for slow, concerted endothermic reaction: Label axes_ reactant, product, transition state, activation energy and AG. Draw reaction coordinate diagram for slow stepwise reaction that favors reactants.
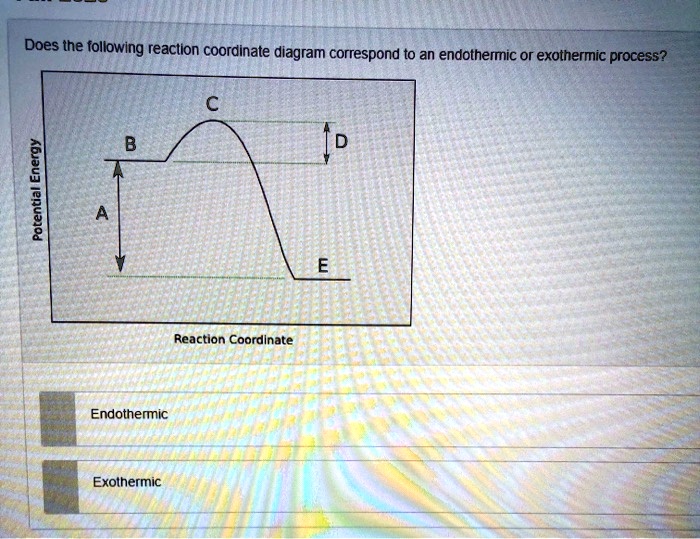
























0 Response to "43 endothermic reaction coordinate diagram"
Post a Comment