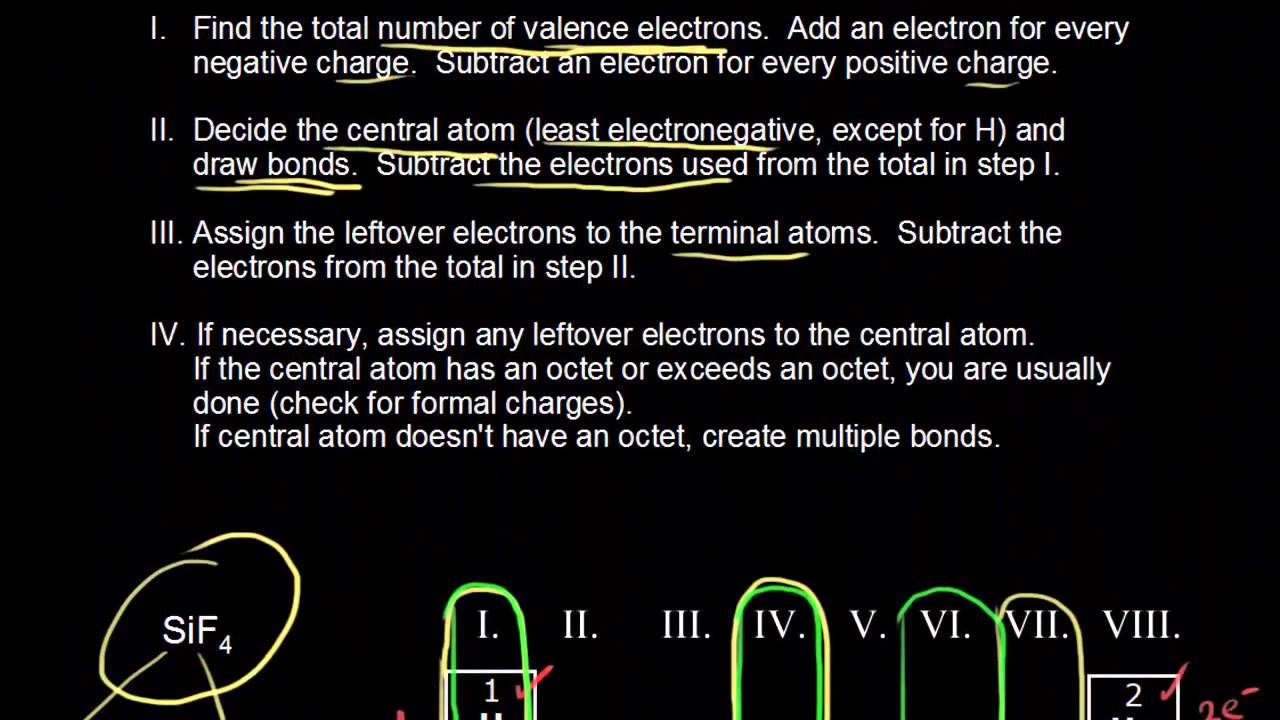
44 in an electron dot diagram, the symbol for an element is used to represent
icp ch. 6 Flashcards - Quizlet in an electron dot diagram, the symbol for an element is used to represent what? ... you see a structural formula in which the symbols for elements are connected by a long dash. you can assume that the chemical bonds in the compound are what? ... the symbol for an element is used to represent what? Which of the following ions would represent the ion of an ... Which two notations represent different isotopes of the same element In an electron dot diagram, the symbol for an element is used to represent Predict the charge that a calcium ion would have.
What does the symbol represent in an electron dot plot? Lewis structures (also called Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms in a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules whether they exist as lone pairs or within bonds. Why don't noble gases easily form compounds?

In an electron dot diagram, the symbol for an element is used to represent
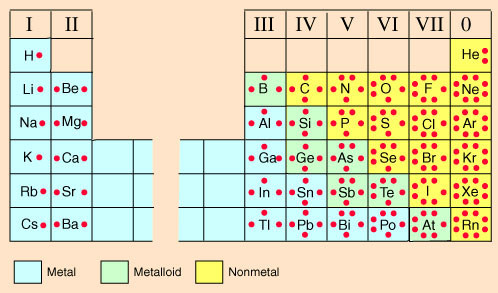
Lewis Dot Diagram Phosphorus - schematron.org A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired. In an electron-dot symbol of an element, the dots are used ... In an electron-dot symbol of an element, the dots are used to represent _____. a. all of the electrons in the atom b. the valence electrons
In an electron dot diagram, the symbol for an element is used to represent. The Lewis Electron-Dot Symbols of Elements Lewis electron-dot symbols work well for the representative elements. A Lewis symbolis a symbol in which the electrons in the valence shell of an atom or simple ion are represented by dots placed around the letter symbol of the element. Each dot represents one electron. Hydrogen Oxygen Chlorine Chloride ion What Goes In The Center Of An Electron Dot Diagram ... A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Solved In an electron dot diagram, the symbol for an ... Expert Answer Transcribed image text: In an electron dot diagram, the symbol for an element is used to represent a. The nucleus b. The nucleus and all electrons C. The nucleus and all valence electrons d. The nucleus and all non-valence electrons. What do the dots in an electron dot formula represent? What do the dots in an electron dot formula represent? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
In an electron dot diagram, the symbol for an element is ... Sep 06, 2015 · In an electron dot diagram, the symbol for an element is used to represent the nucleus and all nonvalance electrons. Log in for more information. Added 9/6/2015 6:48:53 PM. This answer has been confirmed as correct and helpful. What do the dots around a symbol in a Lewis structure ... The dots simply represent electrons. A single dot represents the one electron. A dash typically represents 2 electrons, and typically represents a covalent bond, i.e. shared electron density between two positively charged atomic nuclei. Most of the time we represent the valence electrons only. The inner shell electrons are along for the ride and do not participate in bonding. Trey ~ Science ~ Chapter 6 Flashcards - Quizlet In an electron dot diagram, the symbol for an element is used to represent Nucleus In an electron dot diagram for lithium, carbon, fluorine, and neo which one is the most stable of the elements Neon In the compound MgCl2, the subscript 2 indicates that There are 2 atoms of chlorine List some typical properties of an ionic compound In an electron dot diagram, the symbol for an element is ... Mar 30, 2018 · In an electron dot diagram, the symbol for an element is used to represent the nucleus. For example : As we know that arsenic has '5' valence electrons. So, the symbol (As) is used to represent the nucleus and valance electrons around the is represented by the 'dot'. The Lewis-dot structure of As (arsenic) is shown below. Advertisement Survey
Lewis Structures: Dot Symbols, Diagrams, Examples, Questions How to Draw Electron Dot Formula? We represent an electron dot structure by using the symbol of an element. The dots that represent the valence electrons are added to the chemical symbol of an element in a clockwise manner. We cannot put the dots anywhere around the symbol. Instead, we need to imagine a square around the chemical symbol. What Is The Symbol Of The Element Located In Period 3 With ... What does an electron dot structure show? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. What do the dots on an electron dot diagram represent ... Aug 30, 2019 · Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Electron dot diagrams would be the same for each element in the representative element groups. Solved in a electron-dot symbol of an element , the dots ... Transcribed image text: QUESTION 5 In an electron-dot symbol of an element, the dots are used to represent the electrons that the element will gain when it forms a compound the electron arrangement all of the electrons in the atom only the electrons that will participate in bond formation the valence electrons Previous question Next question
In an electron dot diagram, the symbol for an element is ... In an electron dot diagram, the symbol for an element is used to represent General. In an electron dot diagram, the symbol for an element is used to represent. 861 students attemted this question. Bookmark. Add Comment.
In an electron dot structure of an element the dots are ... 11. _____In an electron-dot structure of an element, the dots are used to represent _____. a. all of the electrons in the atom. b. the valence electrons. c. the complete electron arrangement. d. only the electrons that will participate in bond formation. e. the electrons that the element will gain when it forms a compound.
How does an electron dot diagram work? Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Electron dot diagrams would be the same for each element in the representative element groups. Click to see full answer.
in an electron dot diagram the letter symbol for an ... The symbol in an Electron Dot Diagram is used to represent the nucleus and all the non-valence electrons, The Electron Dot Diagram is also known as the Lewis Dot Symbol. The Lewis Dot Symbol or Electron Dot Diagram, is a symbol of an element with one or more dots representing the valence electrons of an element.
What do the dots in a Lewis dot structure represent ... Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.
In an electron dot diagram the symbol for an element is ... Aug 22, 2014 · "The electron dot diagram is used by scientists to represent electron sharing. The chemical symbol is in the center to represent the nucleus and inner energy level. Dots surrounding the symbol that...
Lewis Symbols and Structures - Chemistry Lewis structure diagram showing lone pairs and bonding pairs of electrons in a molecule or an ion Lewis symbol symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion lone pair two (a pair of) valence electrons that are not used to form a covalent bond octet rule
In an electron-dot symbol of an element, the dots are used ... In an electron-dot symbol of an element, the dots are used to represent _____. a. all of the electrons in the atom b. the valence electrons
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired.
Lewis Dot Diagram Phosphorus - schematron.org A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.




![Solved] Match each element to its electron dot diagram. The ...](https://us-static.z-dn.net/files/d0d/4ae06989353e6d1330fc71c6b1812356.png)
/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)









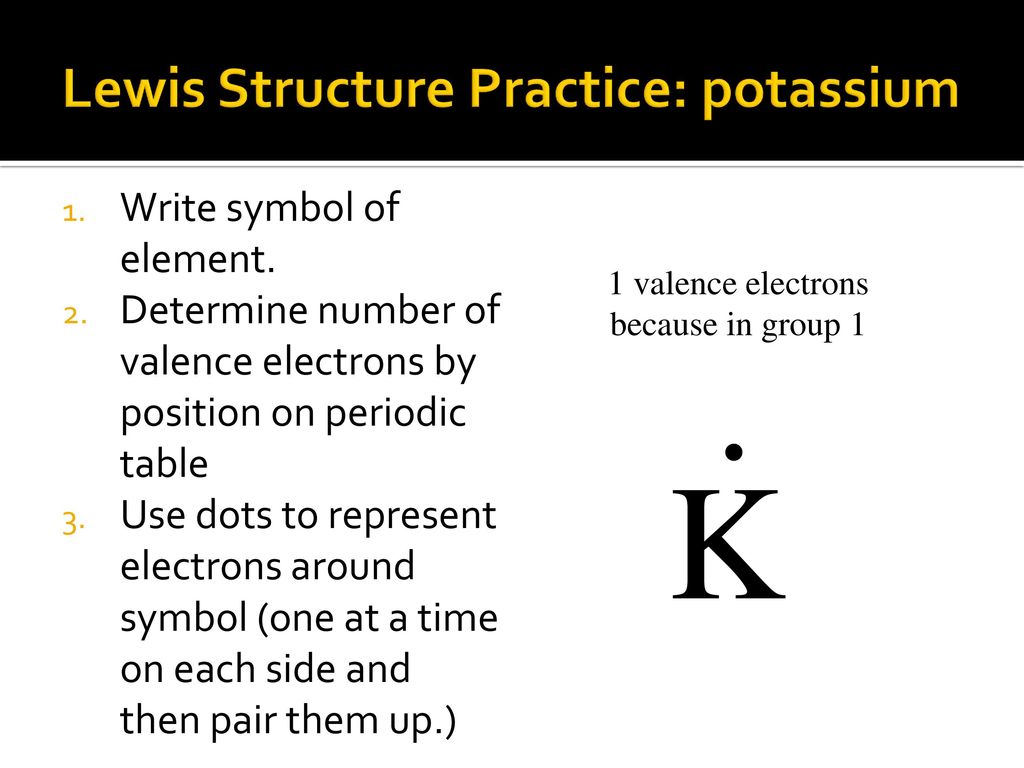

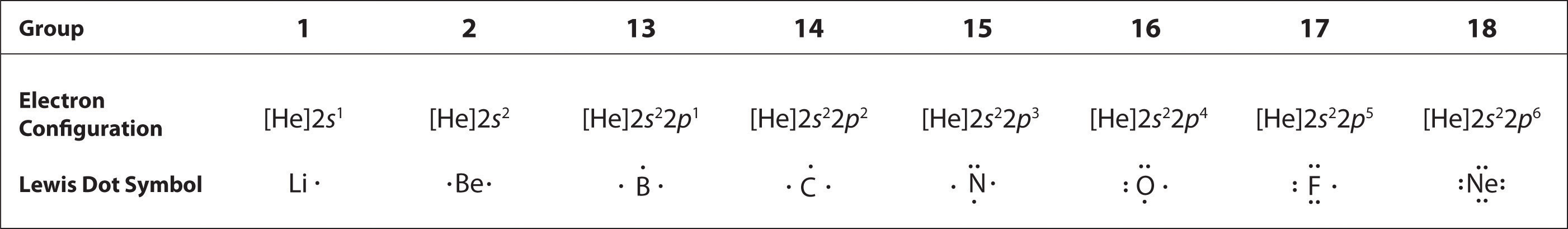


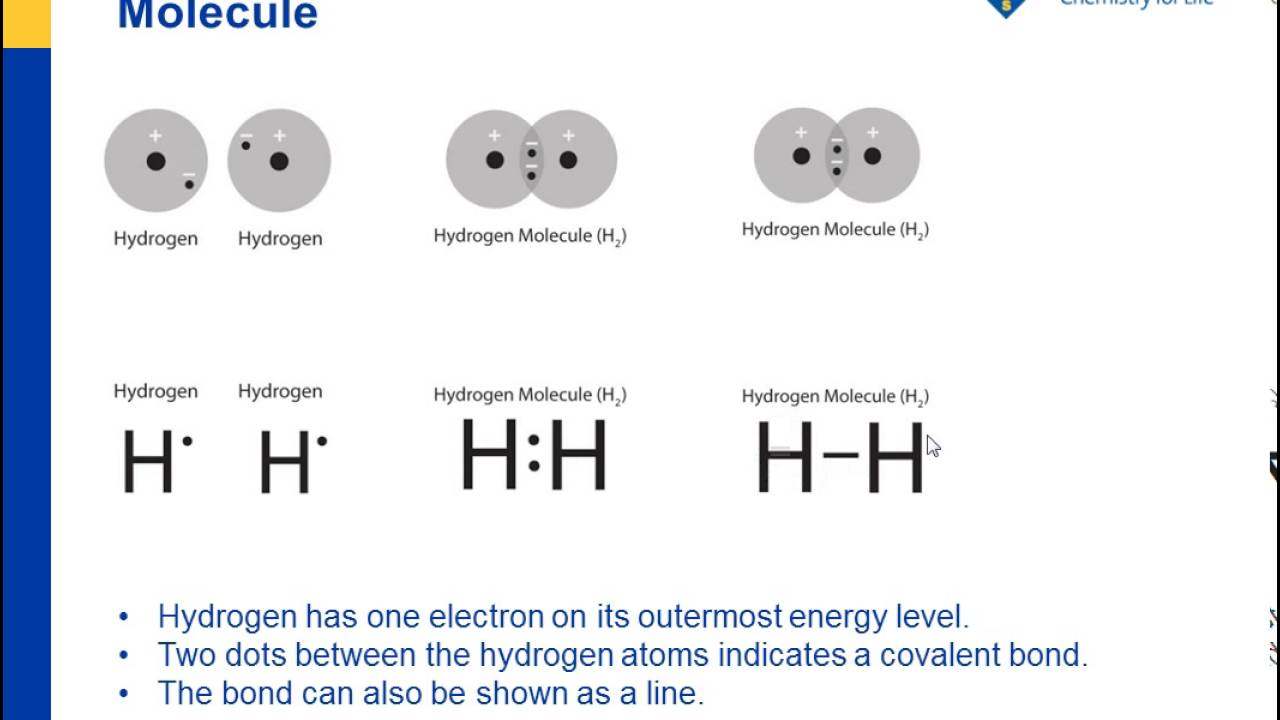





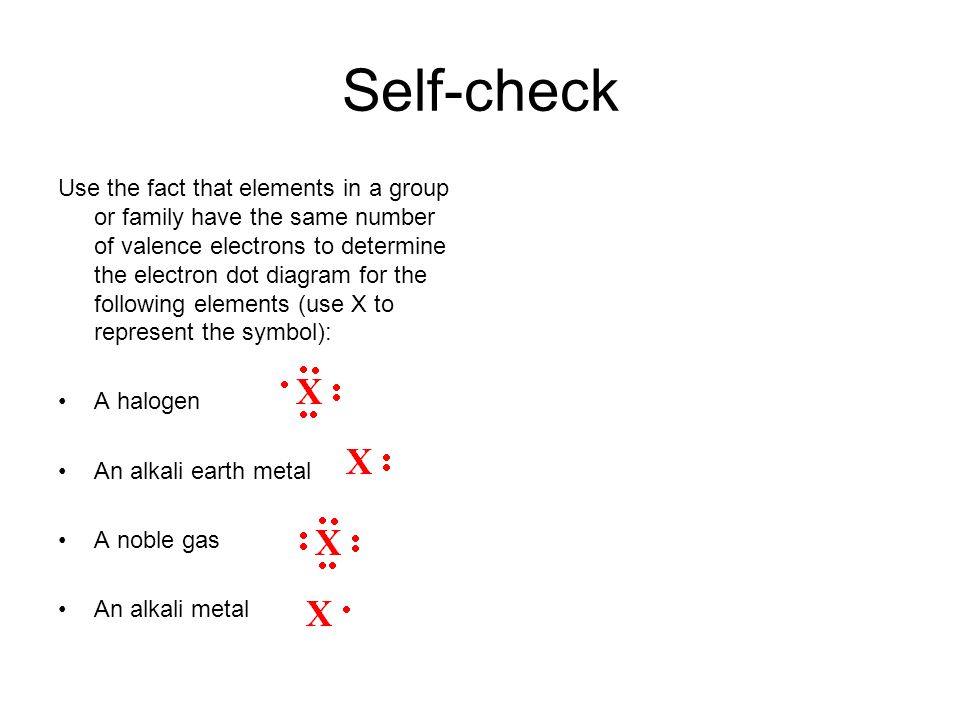


0 Response to "44 in an electron dot diagram, the symbol for an element is used to represent"
Post a Comment