45 lewis dot diagram for pcl3
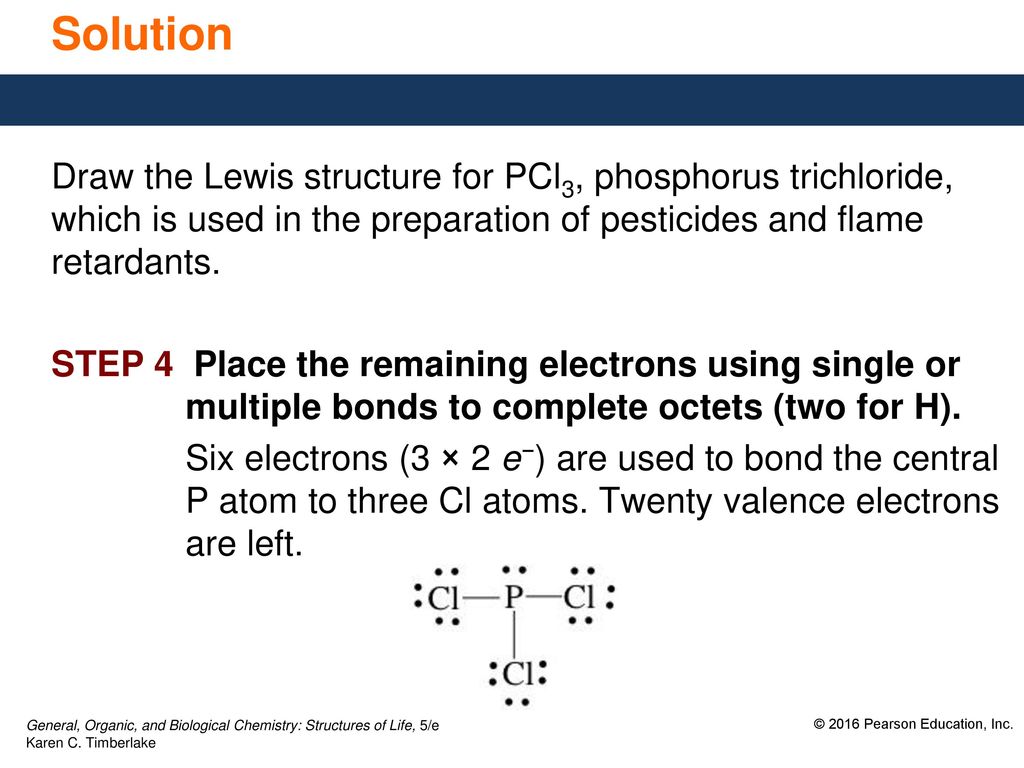
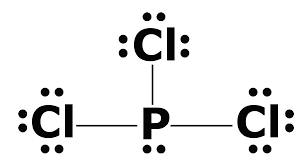
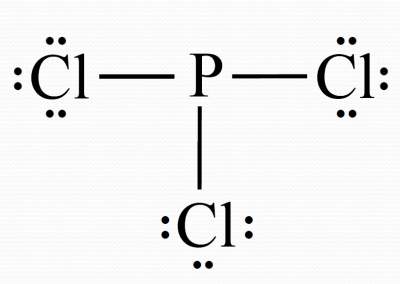
PCl3 lewis structure, molecular geometry, bond angle ... Phosphorous trichloride (PCl3) Lewis structure. Hence, in the above PCl 3 lewis structure, all atoms get a formal charge equal to zero. Therefore, the above lewis dot structure of PCl 3 (Phosphorous trichloride) is most stable and appropriate in nature. What is the compound name of PCl3? - Pvillage.org What is the Lewis structure of PCl3? The structure of PCl3 is a phosphorus with a lone pair (two electrons) and 3 chlorine atoms attached by a single bond where each chlorine has 3 lone pairs. This is also called a Lewis Structure. Photo from Google. What is the Lewis dot structure for PCl3?
How many valence electrons are in the Lewis structure of PCl3? PCl3 lewis structure In this lewis structure of PCl 3, center phosphorus atom has made three single bonds with three chlorine atoms. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs. Also, there are no charges on atoms in PCl 3 lewis structure. What is the Valency of PCl3?
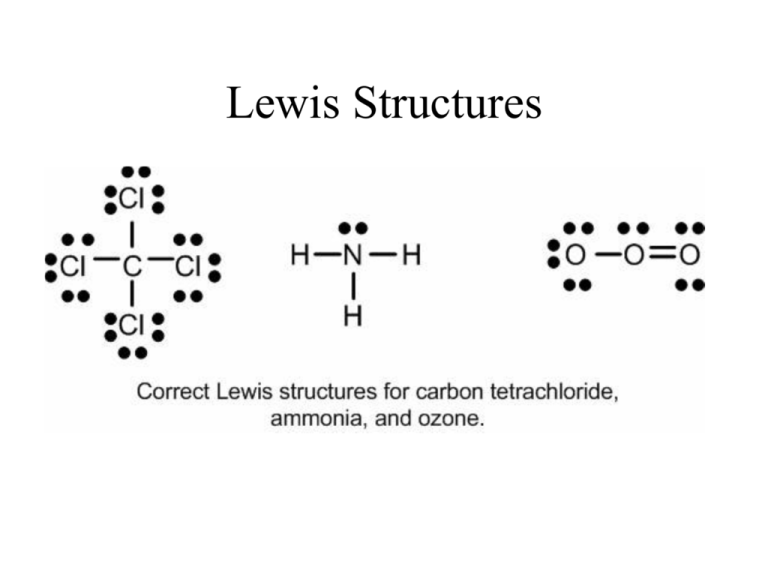
Lewis dot diagram for pcl3
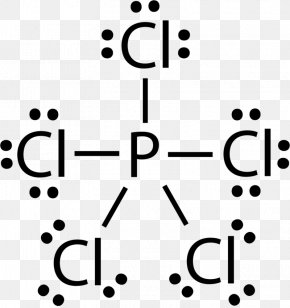
PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories. Lewis Dot Structure of PCl3 (Phosphorous TriChloride ... I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle. Lewis Structures - Lewis Dot Structure | Chem Helps Lewis Dot Structure can be drawn in that way. Bond Degree. It is the number of bonds that join certain atoms in a molecule. If there is only one bond between two atoms, the degree of bond is 1, if there are 2 bonds, the degree of bond is 2, if there are 3 bonds, the degree of bond is 3. Sample: Let us show the Lewis dot structure of the C3H8 ...
Lewis dot diagram for pcl3. What is the Lewis structure of PBr3? - Answers This is the Lewis Dot Structure . this may be the Lewis Structure But i am not 100% :S: ll :S: What is the Lewis structure of AsF5? I think it's similar to the Lewis structure for PCl5. So, if you ... Phosphorus trichloride | PCl3 - PubChem In a Hoechst continuous process, molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorus.The heat of reaction, ca. 10 times the heat of evaporation, keeps the system at its boiling point, and the phosphorus trichloride distills off. Solved a) Draw a valid lewis electron dot Structure for ... Chemistry questions and answers. a) Draw a valid lewis electron dot Structure for phosphorus trichloride (PCl3) accounting for all valence electronsDraw or state the shape of PCl3 moleculesHow many 'lone pairs' does this molecule have?b) Draw a valid lewis electron dot Structure for carbonate ion (CO32-) accounting for all valence electronsDraw ... PCl3 Lewis Structure - How to Draw the Lewis Structure for ... A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the tot...
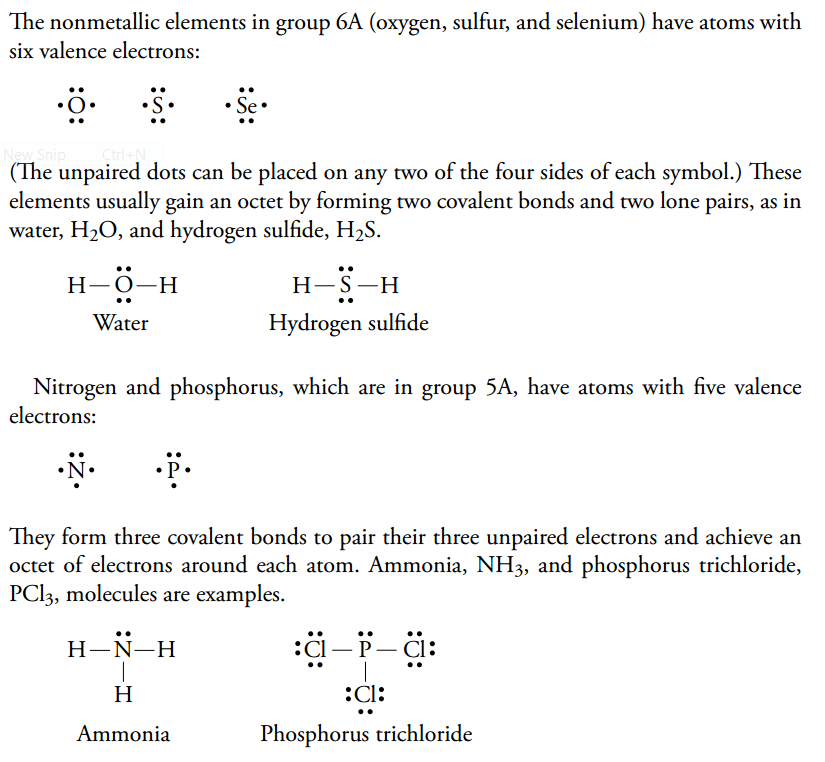
Nitrogen trichloride (NCl3) lewis dot structure, molecular ... Lewis diagram is a representation of how electrons are arranged around individual atoms in a structure. NCl3 lewis structure is the same as the NF3 structure. It contains one nitrogen atom at the center and three chlorine atoms spaced evenly around it. Lewis structure calculator | Lewis structure generator To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. For example, if we want to obtain the Lewis structure of the Sulfate ion, SO4- 2, we must first enter the charge by typing (-2) or by entering -2 in the charge field and pressing the «Add» button. PCl3 Molecular Electron Geometry, Lewis Structure, Bond ... Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas. It is a toxic compound but is used in several industries. Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
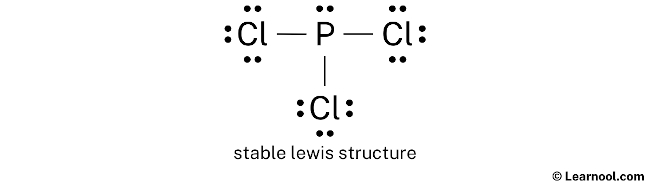
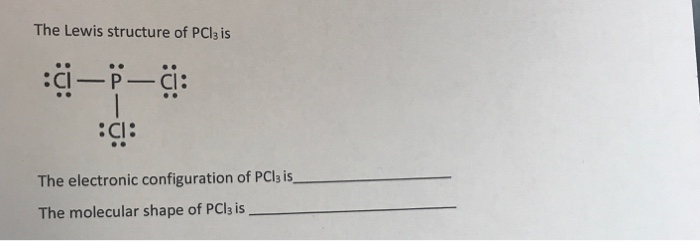
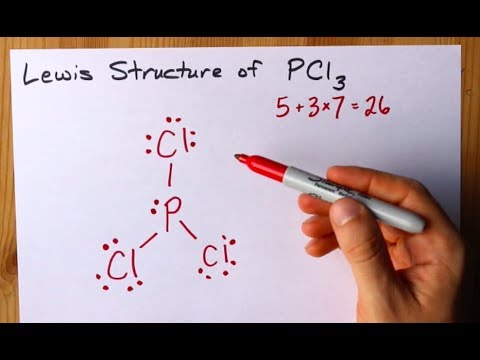
PCl3 Lewis Structure: How to Draw the Dot Structure for PCl3 Let's do the Lewis structure for PCl3. Phosphorus, on the periodic table, is in group 5, it has 5 valence electrons. Chlorine, group 7, but we have three of those so we have 5 plus 7 (times 3 is 21) is 26 valence electrons. We'll put the Phosphorus in the center and then we'll put the Chlorines around it, just like that. PCl3 Lewis Structure, Hybridization, Molecular Geometry ... We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. Another simple formula can also give us the hybridization of PCl3. PDF ap07 chemistry q6 - College Board One point is earned for a correct Lewis diagram (can be done with dots or lines). (b) On the basis of the Lewis electron-dot diagram that you drew in part (a), predict the molecular geometry of the IF 3 molecule. T-shaped One point is earned for the molecular geometry consistent with the Lewis diagram in part (a). (c) In the SO 2 Lewis Structure Pcl3 [GK3J1E] You can watch me draw the Lewis Dot Diagram for PCl3 here. Lewis, who described them in a 1916 article titled, "The Atom and the Molecule. How many pi bonds in the formula. In a Hoechst continuous process, molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. Lets do the lewis structure for pcl3.
What is the electron dot structure for PCl3 class 11 ... Hint: Electron dot structures or diagrams are also called Lewis structure. They are the representation of the total valence electrons in any atom or molecule. For polyatomic species, the valence electrons of each atom are calculated and added, then arranged on the individual atoms to obtain an electron dot structure.
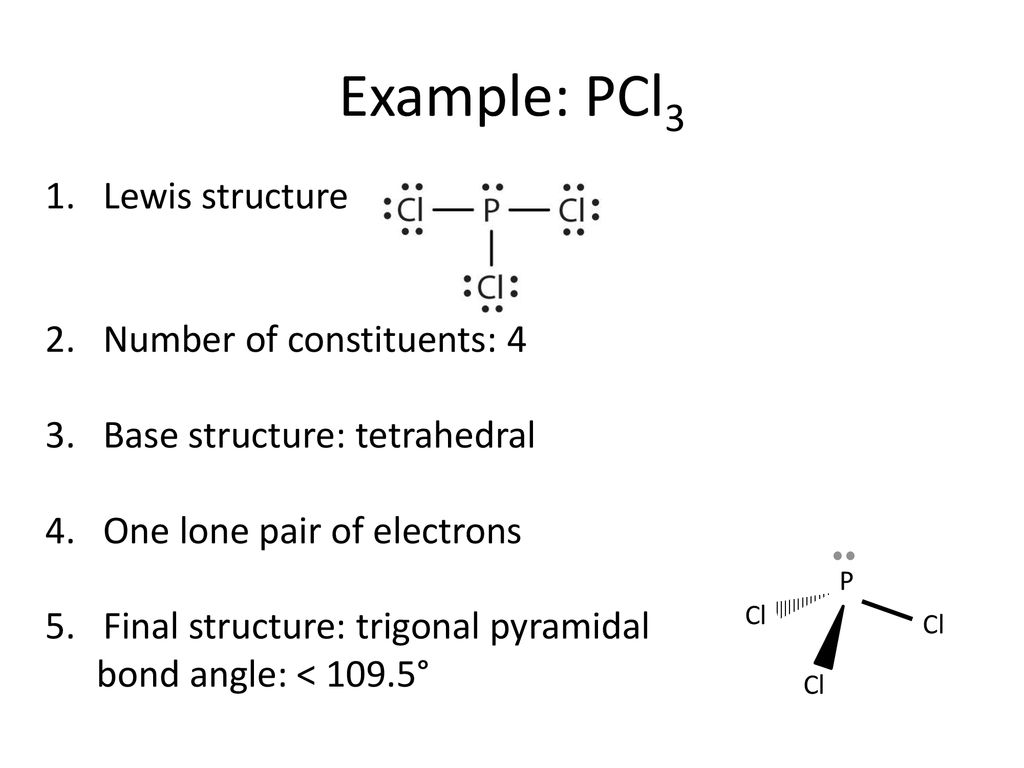
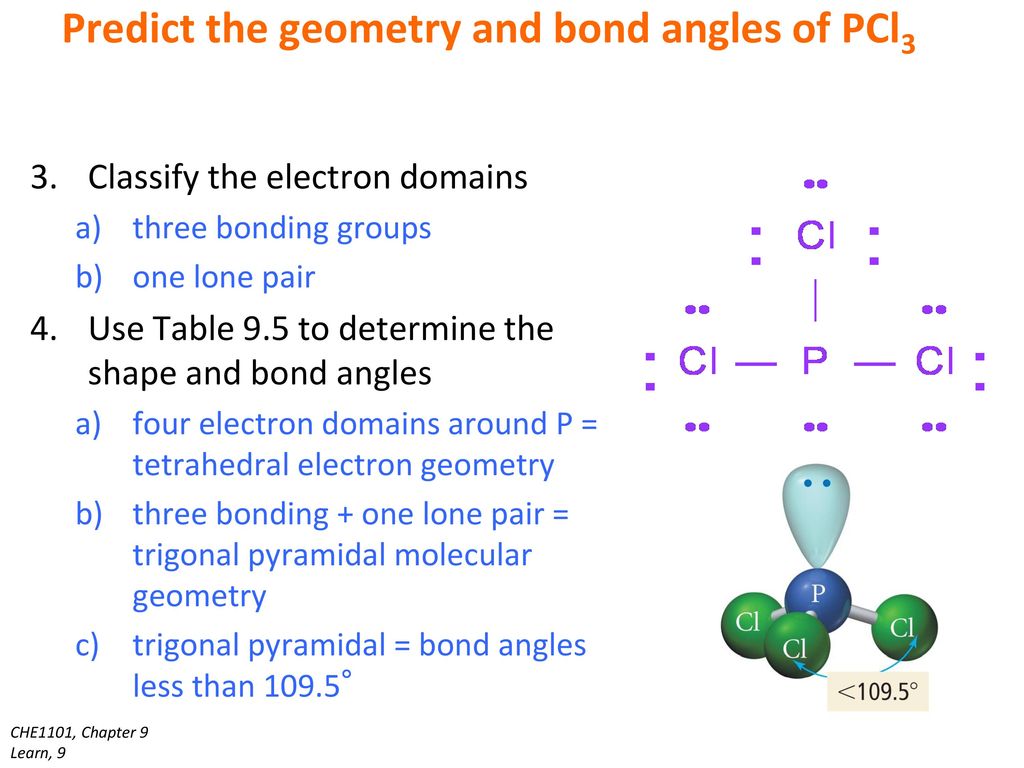
How to draw PCl3 Lewis Structure? - Science Education and ... In the PCl3 Lewis structure diagram, the phosphorus atom can be the center atom of the molecule. As a result, central phosphorus in the PCl3 Lewis structure, with all three chlorine atoms arranged in a trigonal pyramidal geometry. Add valence electrons around the chlorine atom, as given in the figure.
Lewis Dot Structure For Phosphorus Trichloride - drawing easy Lewis structure pcl3 molecular electron geometry, lewis structure, bond angles and hybridization posted by priyanka 07 feb phosphorus trichloride is made up of one phosphorus atom and three chlorine atoms, having a chemical formula of pcl3. Drawing pcl3 lewis structure is very easy to by using the following method. Source: kovodym.blogspot.com ...
What is the Lewis dot structure for PCl3? - handlebar ... We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. How is the phosphorus trichloride ( PCl3 ) made?
Lewis Structure For Pcl3 - DiviNewsMedia.com The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. One is phosphorous P and the second is chlorine Cl. These Atoms can exceed 8 surroundings electrons in the structure. Click and drag the molecle to rotate it. In the Lewis structure for PCl 3 there are a total of 26 valence electrons.
How is the electron dot structure of PCl3 determined? - Quora Answer: Count total valence electrons. Chlorine 3x7=21; Phosphorus 1+5=5; 21+5=26. This tells us that these are all the electrons we have available to use in our structure. Next we write down P as the central atom and connect a single bond to each Cl. Each bond counts as two electrons so now we h...
Lewis Dot of Phosphorous Trichloride PCl3 70 More Lewis Dot Structures. From- . PCl 3 is important indirectly as a precursor to PCl 5, POCl 3 and PSCl 3. which in turn enjoy ...
Lewis Structures - Lewis Dot Structure | Chem Helps Lewis Dot Structure can be drawn in that way. Bond Degree. It is the number of bonds that join certain atoms in a molecule. If there is only one bond between two atoms, the degree of bond is 1, if there are 2 bonds, the degree of bond is 2, if there are 3 bonds, the degree of bond is 3. Sample: Let us show the Lewis dot structure of the C3H8 ...
Lewis Dot Structure of PCl3 (Phosphorous TriChloride ... I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle.
PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories.


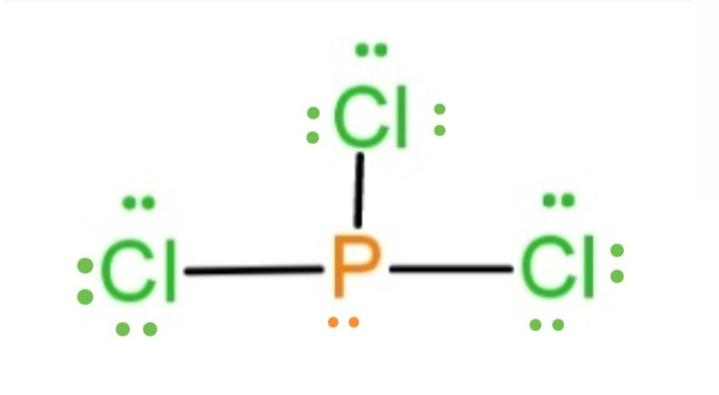
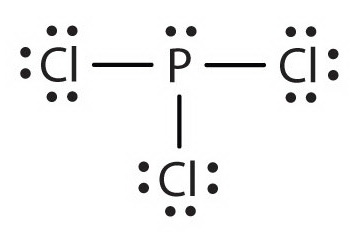





















0 Response to "45 lewis dot diagram for pcl3"
Post a Comment