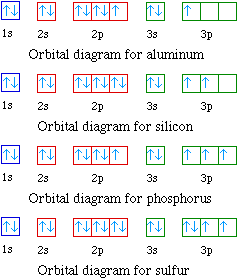
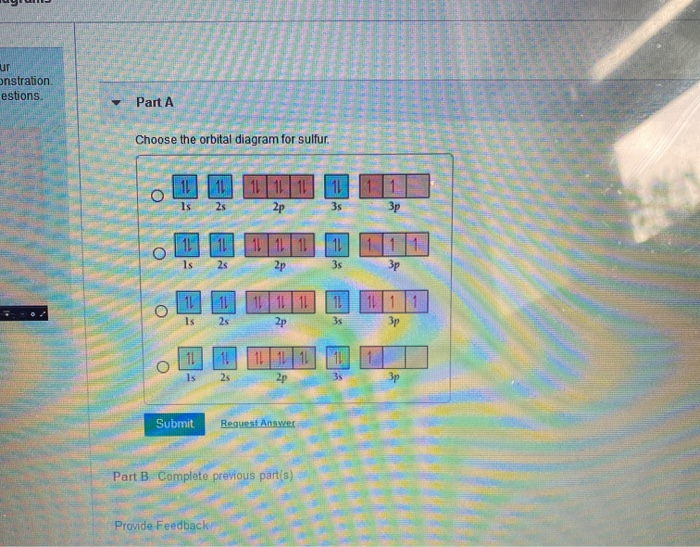
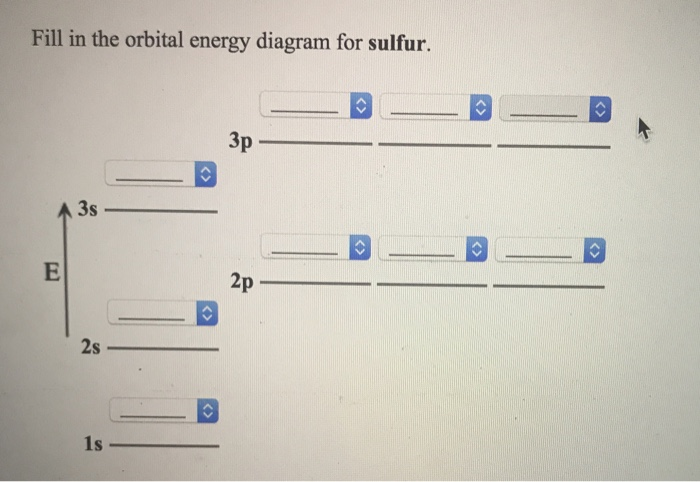
45 orbital diagram for sulfur
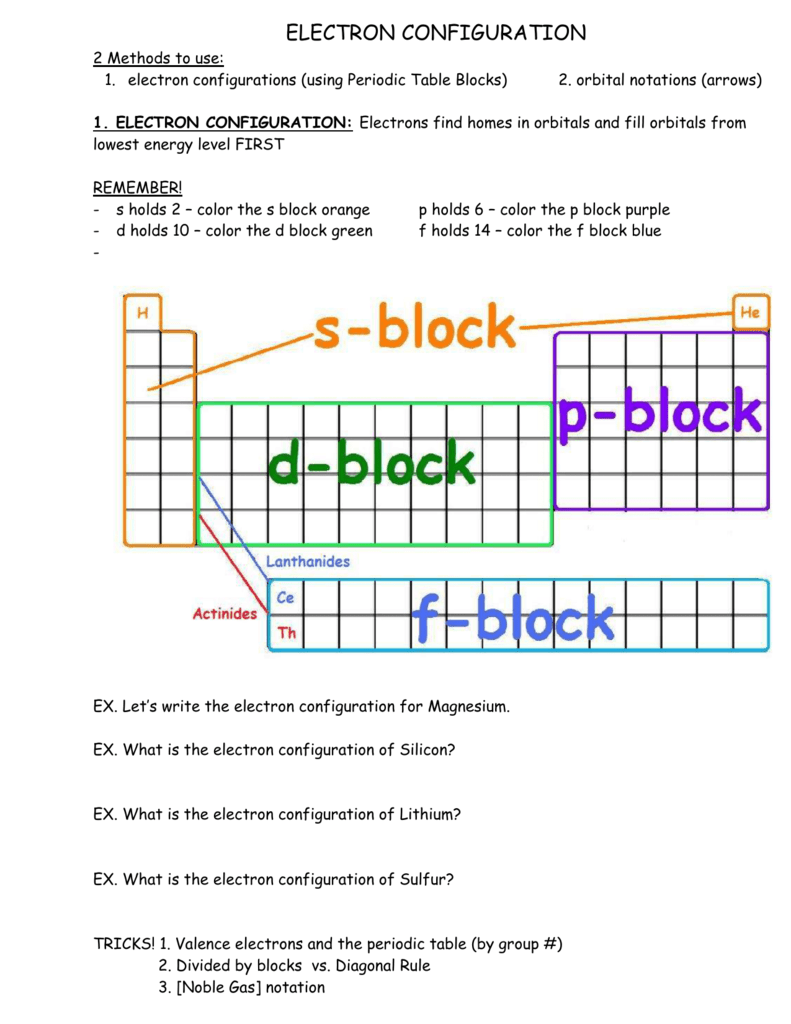
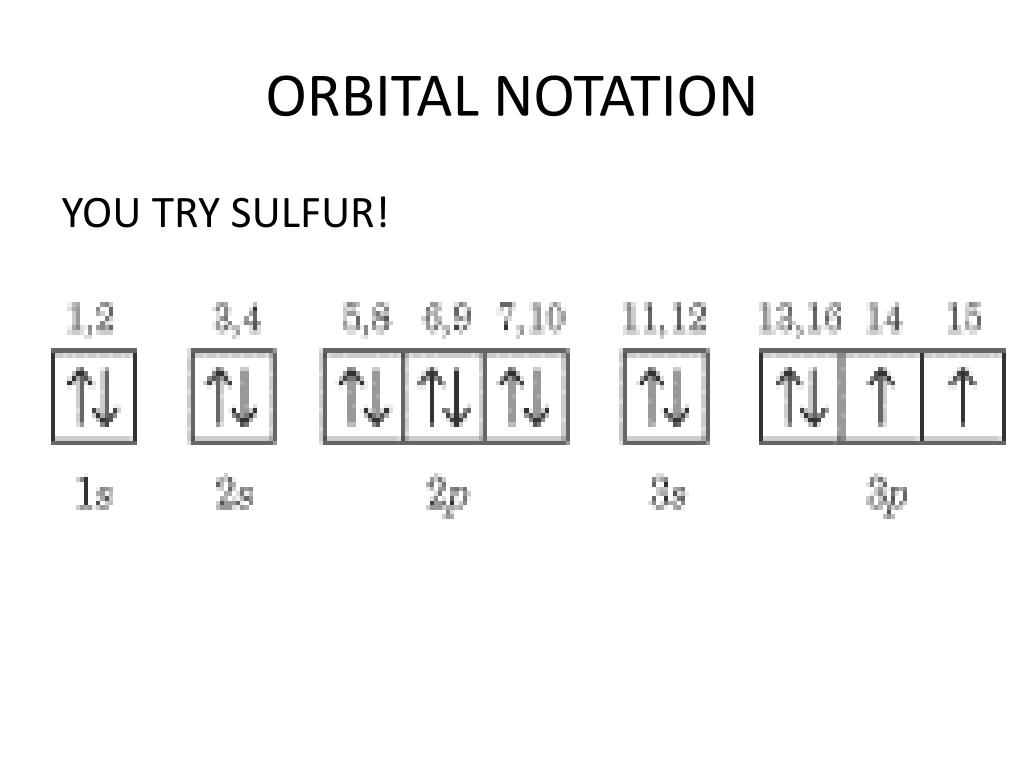
Electron Configuration Boxes - 9 images - 5 5 orbital ... orbital box diagram for sulfur. Electron Configuration Boxes. Here are a number of highest rated Electron Configuration Boxes pictures upon internet. We identified it from trustworthy source. Its submitted by management in the best field. We receive this kind of Electron Configuration Boxes graphic could possibly be the most trending topic ... How to Write an Electron Configuration - ChemTalk In an orbital diagram, orbitals are represented as boxes and electrons are represented by arrows (↑ or ↓), with two electrons occupying each orbital/box. Orbitals are labeled according to their principle energy levels and sublevels (1s, 2p, etc..). Helium, with two electrons in the 1s orbital has the following orbital diagram.
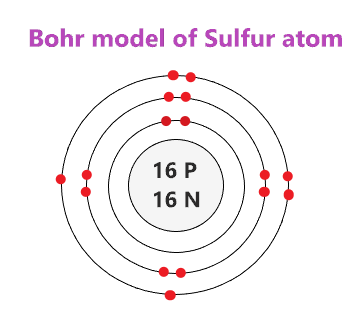
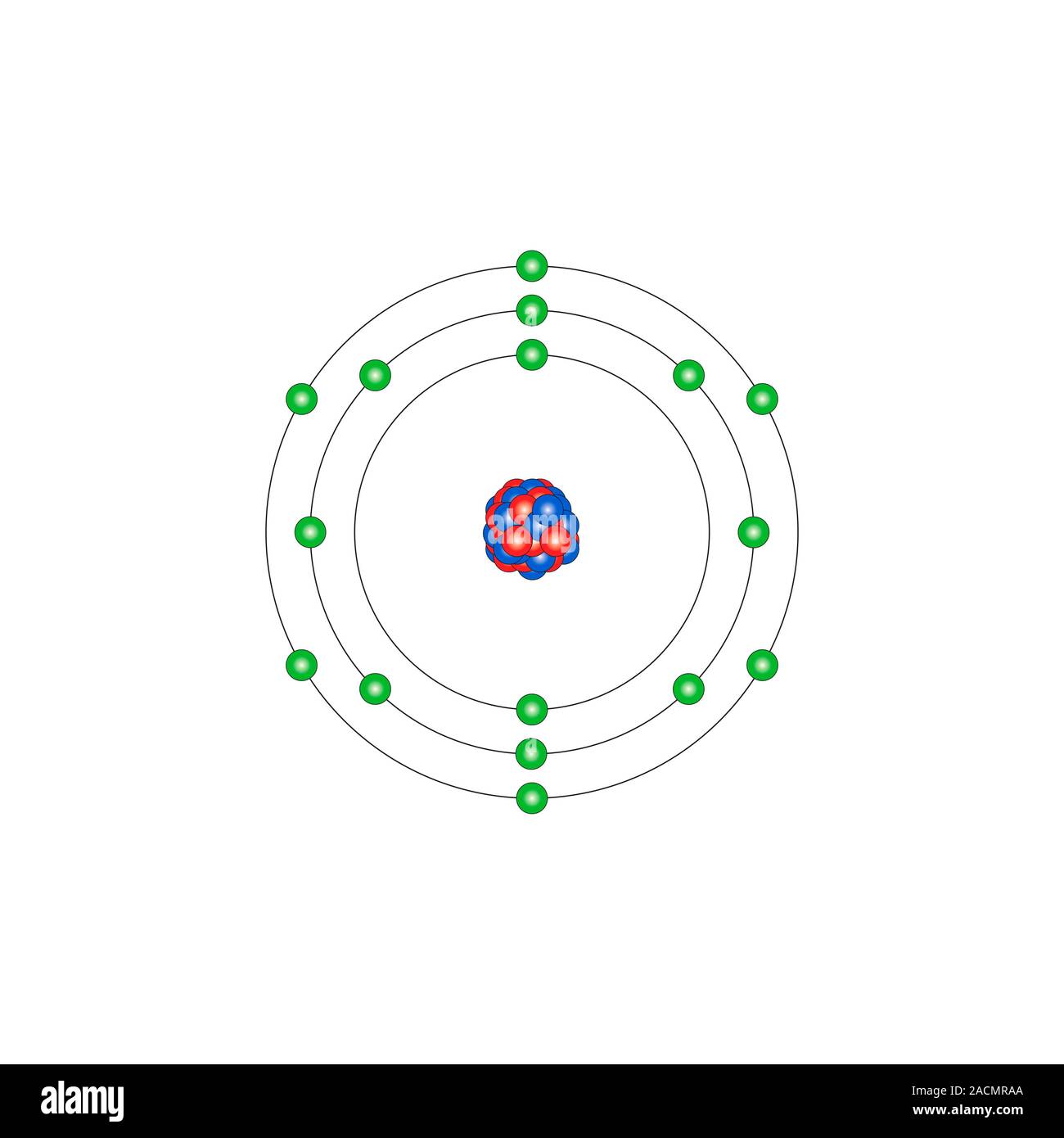
40 orbital diagram for sulfur - Wiring Diagrams Manual Below is the electronic diagram of the Sulfur atom Distribution of electrons over energy levels in the S atom. 1-st level (K): 2. 2-st level (L): 8. Oxygen (O) electron configuration and orbital diagram Oxygen (O) is the 8th element in the periodic table and its symbol is 'O'.

Orbital diagram for sulfur
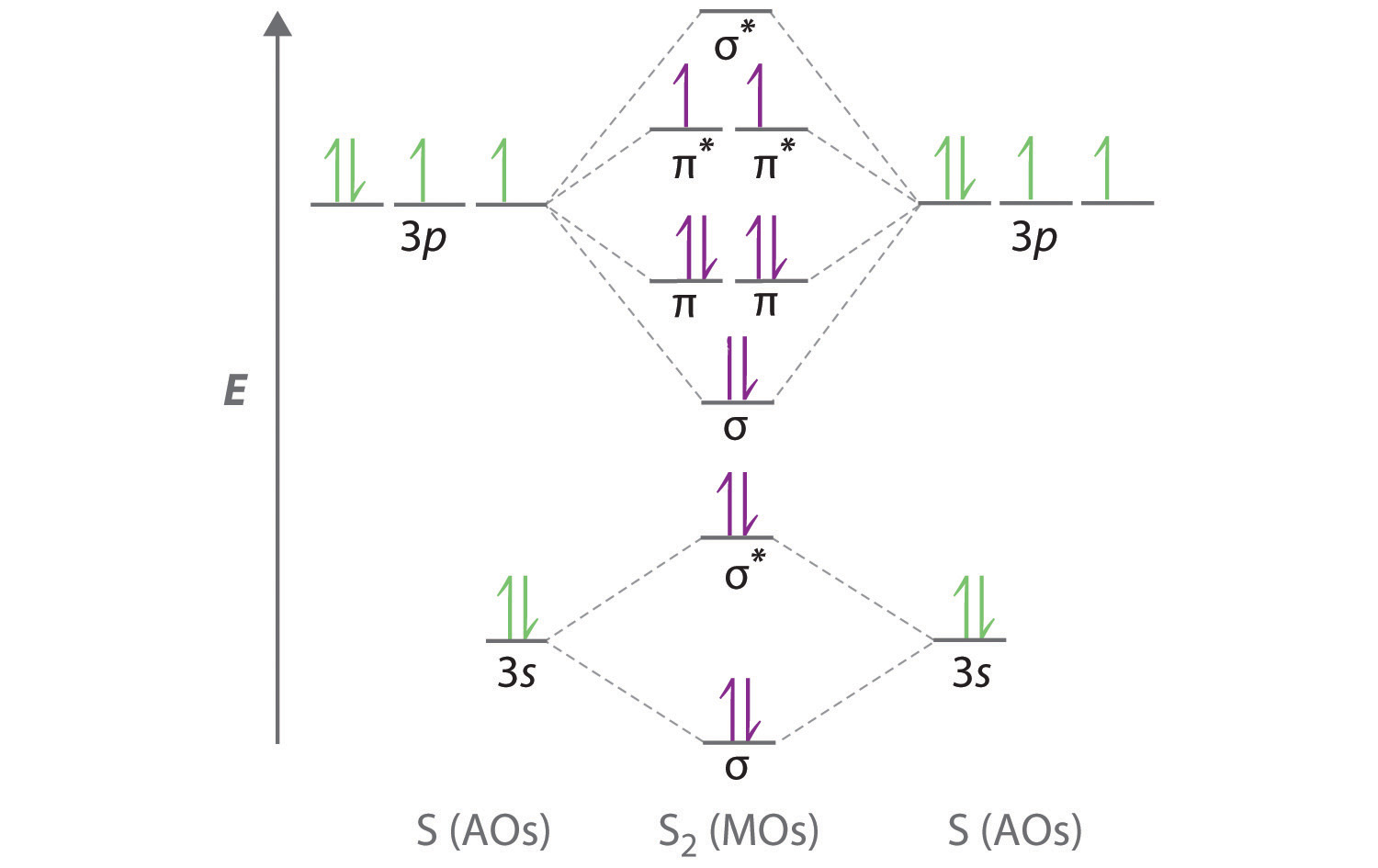
Sulfur (S) - Periodic Table (Element Information & More) So the last electron of sulfur enters the p-subshell or p-orbital. Hence, sulfur is the p-block element. 10 Interesting facts about Sulfur. Interesting facts about sulfur element are mentioned below. ... Orbital Diagram of All Elements (Diagrams given Inside) 4.10: Second-Row Diatomic Molecules - Chemistry LibreTexts Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence electrons (7 from each F atom), all of the energy levels except the highest, are filled. This diagram shows 8 electrons in bonding orbitals and 6 in antibonding orbitals, resulting in a bond order of 1. 闪电代写 -代写CS作业_CS代写_Finance代写_Economic代写_Statistics代写_代码代做 ... Question 2 (10 marks). The diagrams below show three proposed Lewis structures for the chlorate ion, ClO3 - . (a) Without referring to the diagrams, calculate the number of valence electrons in ClO3 - . (b) Circle the correct answer (A, B or C) for each of the following questions in relation to the Lewis
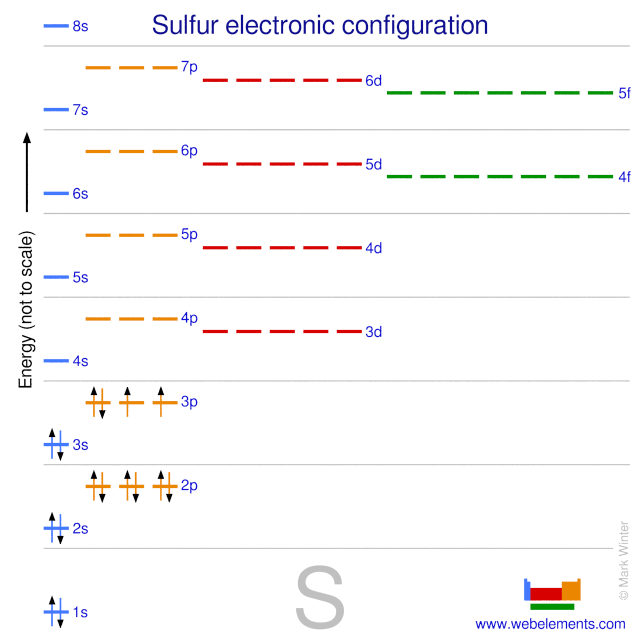
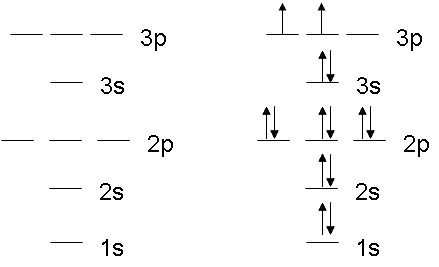
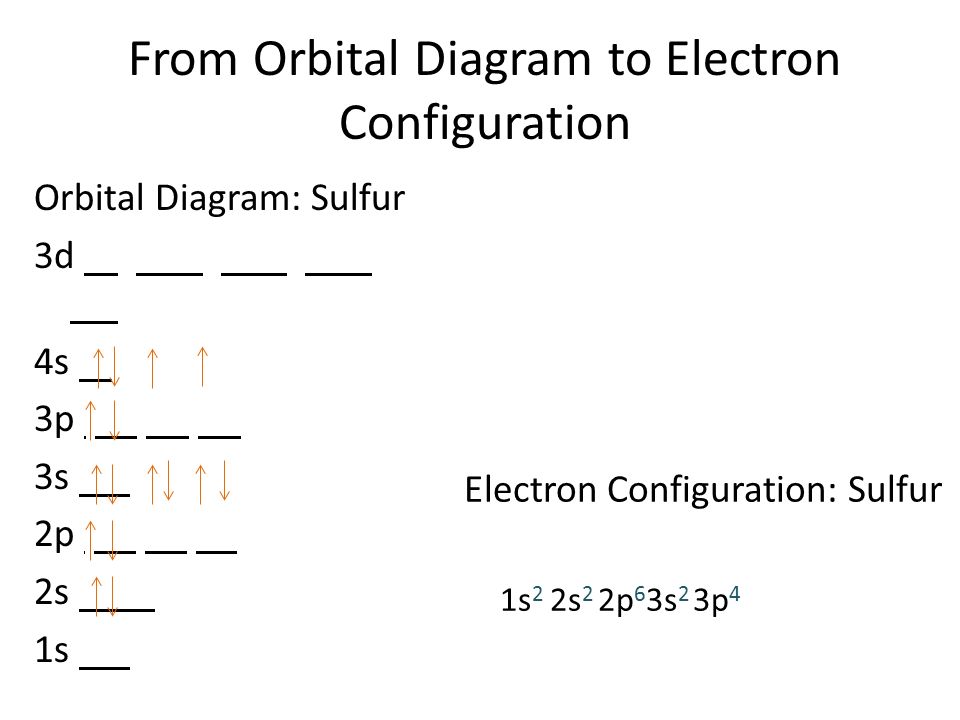
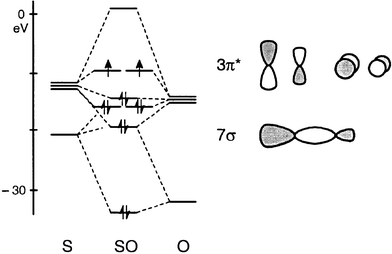
Orbital diagram for sulfur. write the electron configuration and draw the orbital ... Write the electron configuration and draw the orbital diagram of each of the following atoms. scandium gallium silver sulfur lithium 1 See answer Comp Compu Comput Compute Computer Advertisement Advertisement Brainly1215 Brainly1215 Qwertyuiop Asdfghjkl Zxcvbnm anong sagut yan SO2 - A Detailed Discussion Molecular Orbital Diagram of SO 2. We can see that the AO of sulfur on the left associates with the AO of oxygen on the right. Notably, all the orbitals are full of 18 electrons, following the proper rule. Certain non-bonding orbitals are also present there. Furthermore, in the case of SO 2, the antibonding orbitals are unoccupied. Latest Info on SO2 Molecular Geometry - Education Is Around SO2 Molecular Orbital Diagram. A molecular orbital layout offers us an idea concerning how the atomic orbitals of two various atoms can fuse and generate a brand-new orbital. This additional helps us discover the bond order, bond size, and bond toughness of any compound. CHEM 101 - Lecture 5 - Gonzaga University Let us use our orbital energy level diagram to predict the electron configuration of sulfur. The atomic number of sulfur is 16, so there will be 16 electrons in a neutral sulfur atom. We start filling in electrons in our diagram (refer to the figure below, panel (i)) according to the aufbau principle, starting with the lowest energy orbital.
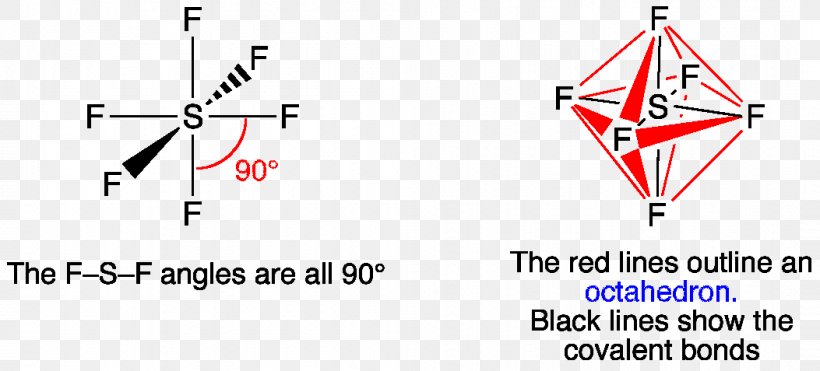
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity. July 23, 2021. Posted by Priyanka. 16 Apr. Sulfur hexafluoride or SF6 is an inorganic, greenhouse gas. It is non-flammable, odourless, and colourless, and is an excellent insulator. It is a hypervalent octahedral molecule that has been an interesting topic of conversation among ... Show the orbital-filling diagram for S (sulfur). Stack the ... Click within the orbital to add electrons. Write the condensed electron. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Calculate the wavelength (in nm) of the red light emitted ... Write the orbital diagram for sulfur and determine the number of unpaired electrons. 15. Write the orbital diagram for Ar and determine the number of unpaired electrons. 16. Write the electron configuration for Ge. Identify the valence electrons and the core electrons. 17. On the basis of periodic trends, choose the larger atom in each pair (if ... Write the electron configuration and orbital arrow diagram ... Junior High School. answer. answered. Write the electron configuration and orbital arrow diagram of the following elements: 1.Sulfur. (S) 2.Gallium. (Ga) 3.
11.8: Molecular Orbital Theory- Electron Delocalization ... Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ... Orbital Diagram For Calcium (Ca) | Calcium Electron ... The 6 electrons will go to the 2p orbital, and the next 2 electrons will place with 3s orbital, and now we have only 8 electrons in which 6 electrons will go with 3p orbital, and the last 2 electrons will be with 4s orbital. So, we have Calcium Electron Configuration is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s². So, the configuration helps to know the ... Hydrosulfuric Acid | Basic information | UO Chemists The lower energy levels are much more stable than, the higher energy levels, and electrons feel very good in the lower energy and stable energy levels. In the molecular orbital diagram of H2S, a heteronuclear atom, one side of the energy level is Hydrogen, and one is sulfur. Valence Electrons Chart for All Elements (Full Chart Inside) Valence electrons in Sulfur (S) 6: 17: Valence electrons in Chlorine (Cl) 7: 18: Valence electrons in Argon (Ar) 8: 19: Valence electrons in Potassium (K) 1: 20: Valence electrons in Calcium (Ca) 2: 21: Valence electrons in Scandium (Sc) 3: 22: Valence electrons in Titanium (Ti) 4: 23: Valence electrons in Vanadium (V) 5: 24: Valence electrons ...
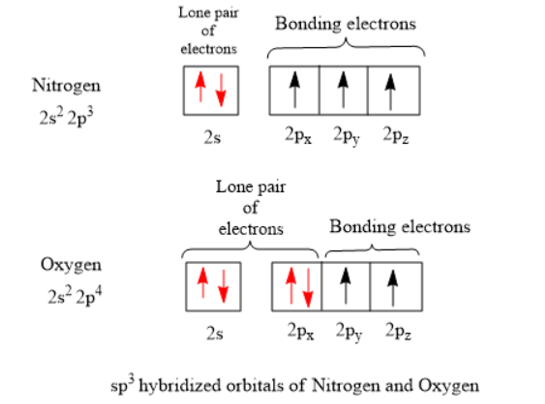
(Get Answer) - Bromine pentafluoride Electron Geometry ... Complete the following table. VSEPR Nvalence shell Valence Bond Theory Nitrogen's orbital diagram Hybridized orbital diagram Nitrite ion 2s 2p Lewis structure Orbitals used in hybridization 2p # + + =N- 0: # 2s+2p+2p Lone : 1 e groups: 3 e groups geometry: trigonal planar -O orbital diagram Sp # # # + =O orbital diagram Bond description for NO ...
What Is The Molecular Shape Of A Hydrogen Sulfide, H2S ... The molecular orbital diagram of H2S have the right to be described in the adhering to way. This is the MO diagram of H2S. The left-hand side will contain the atom orbitals that sulfur i.e 3s2 3px2 3py1 3pz1. And top top the right-hand side, there will certainly be atom orbitals of hydrogen.
14+ Orbital Diagram For Sulfur | Robhosking Diagram 14+ Orbital Diagram For Sulfur. Click within the orbital to add electrons. The aufbau principle tells us that the first energy level (k shell) containing the 1s orbital was completed with the last sulfur : Write the electron configurations for cobalt and lead. Combine the two sodium valence atomic a sulfur has a ne3s23p4 valence electron ...
9.7: Molecular Orbitals Can Be Ordered ... - LibreTexts Exercise 9.7.2. Use a qualitative molecular orbital energy-level diagram to predict the electron configuration, the bond order, and the number of unpaired electrons in the peroxide ion (O 22− ). Answer. ( σ 2 s) 2 ( σ 2 s ⋆) 2 ( σ 2 p z) 2 ( π 2 p x, y) 4 ( π 2 p x, y ⋆) 4 bond order of 1; no unpaired electrons.
Show the orbital-filling diagram for S (sulfur). Stack the ... Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Show the orbital-filling diagram for Br (bromine).
闪电代写 -代写CS作业_CS代写_Finance代写_Economic代写_Statistics代写_代码代做 ... Question 2 (10 marks). The diagrams below show three proposed Lewis structures for the chlorate ion, ClO3 - . (a) Without referring to the diagrams, calculate the number of valence electrons in ClO3 - . (b) Circle the correct answer (A, B or C) for each of the following questions in relation to the Lewis
4.10: Second-Row Diatomic Molecules - Chemistry LibreTexts Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence electrons (7 from each F atom), all of the energy levels except the highest, are filled. This diagram shows 8 electrons in bonding orbitals and 6 in antibonding orbitals, resulting in a bond order of 1.
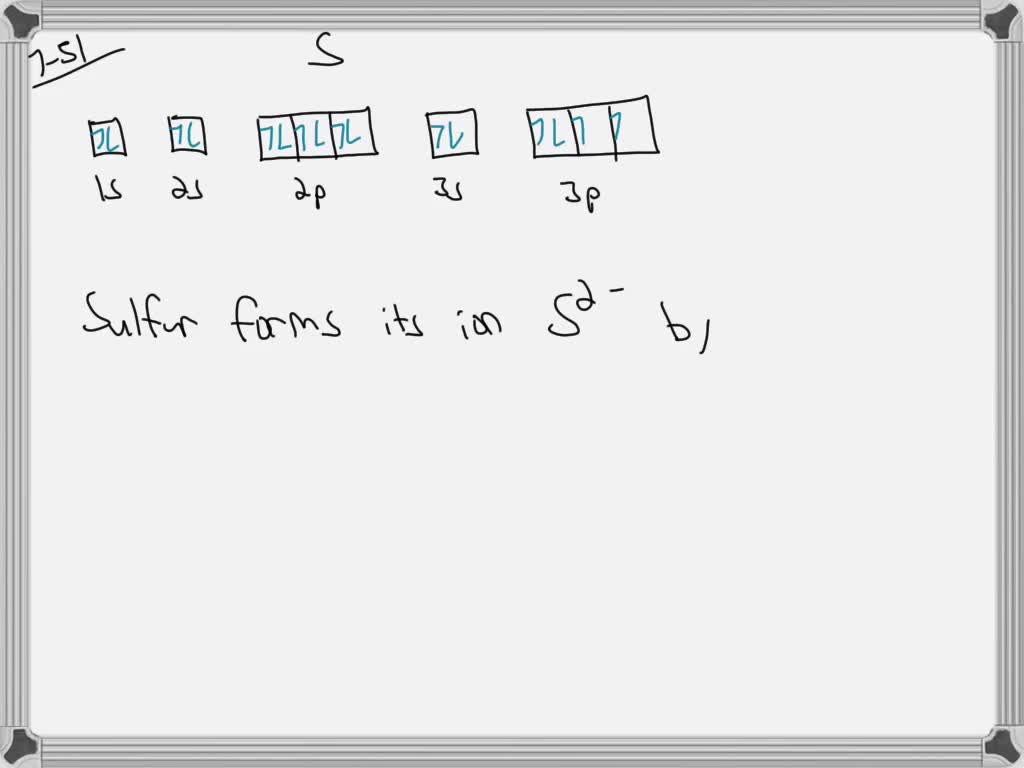
explain the puckered ring structure of s_8 and the arrangement of these in rhombic and monoclinic su
Sulfur (S) - Periodic Table (Element Information & More) So the last electron of sulfur enters the p-subshell or p-orbital. Hence, sulfur is the p-block element. 10 Interesting facts about Sulfur. Interesting facts about sulfur element are mentioned below. ... Orbital Diagram of All Elements (Diagrams given Inside)








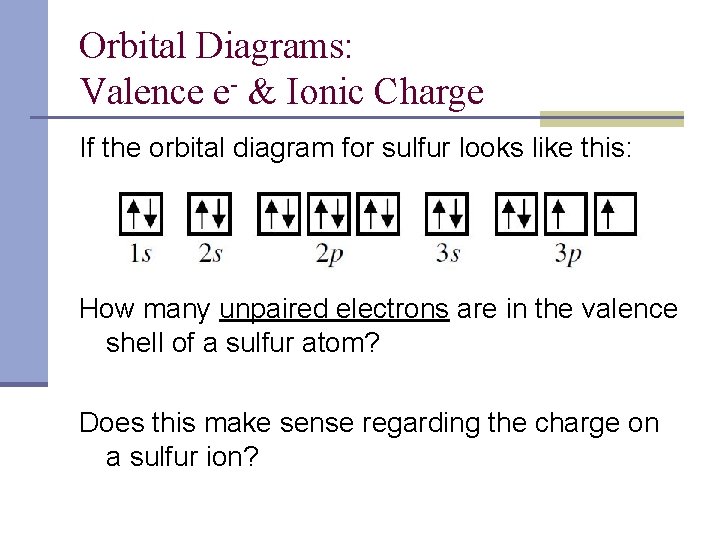
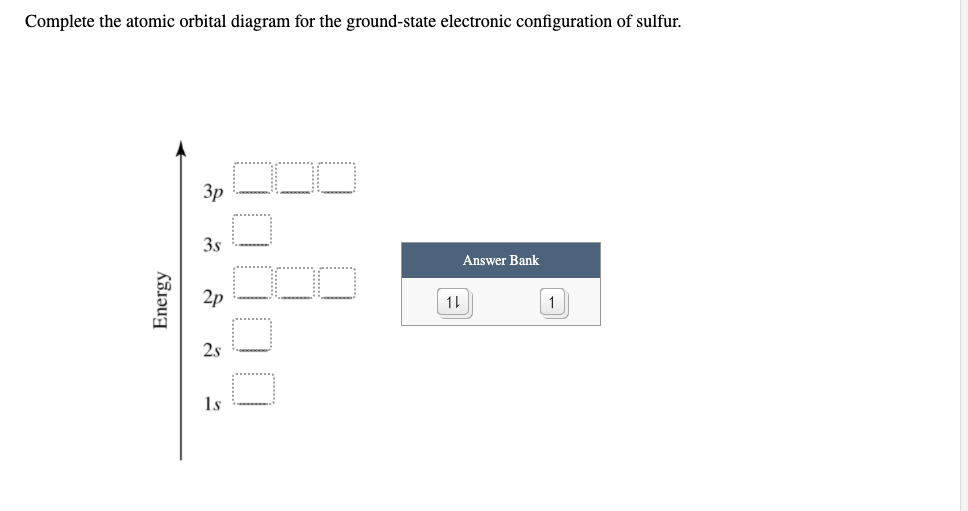
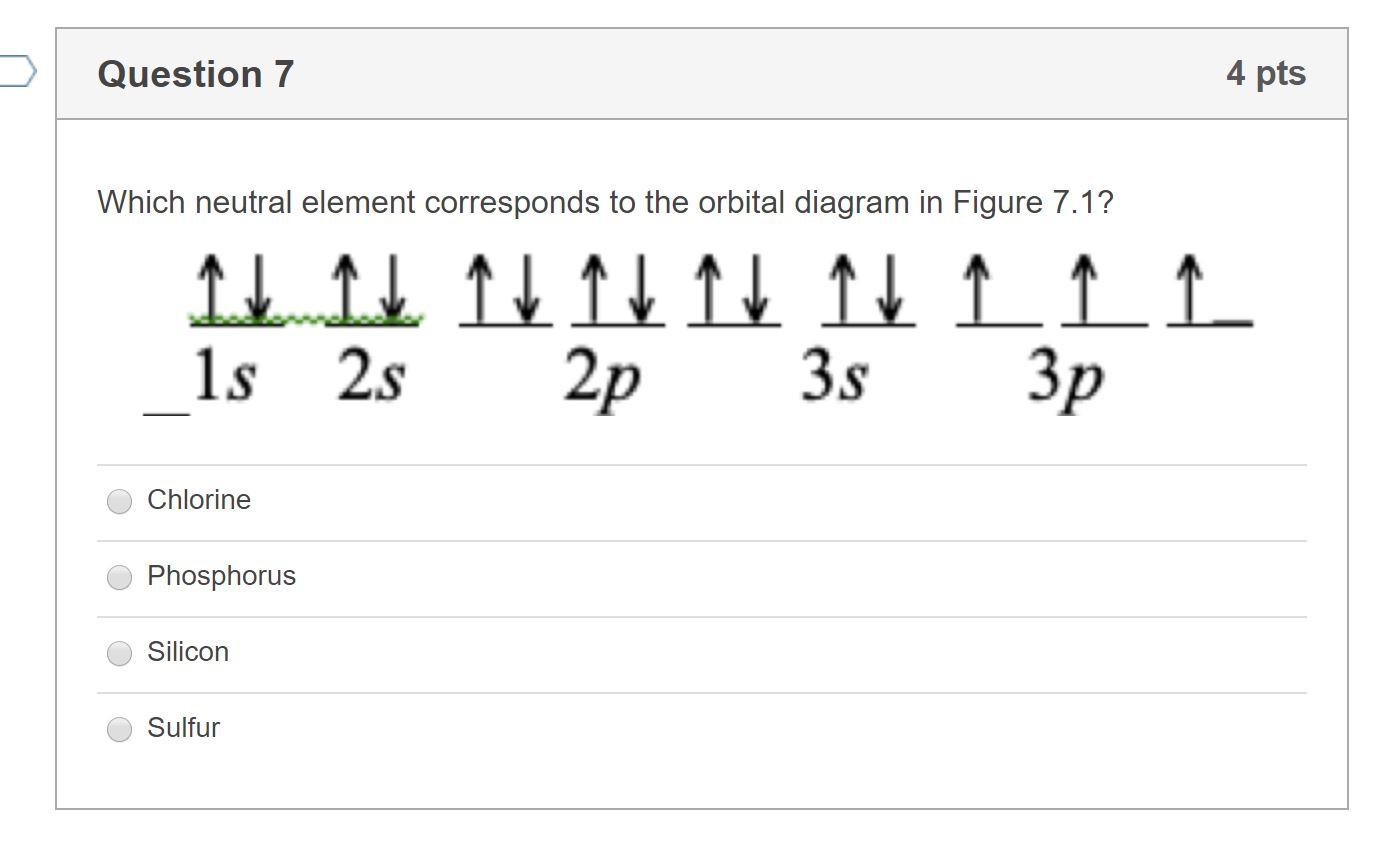










0 Response to "45 orbital diagram for sulfur"
Post a Comment