42 co2+ orbital diagram
The orbital filling diagram for carbon. Again, we start with the electron configuration, which is 1s²2s²2p². As we've seen, this means that there are 2 electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbitals. This is shown like this: Now let’s learn the last topic of this article, the molecular orbital diagram of SO2. SO2 Molecular Orbital Diagram. The molecular orbital diagram of SO2 is attached below: SO2 Molecular Orbital Diagram. A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital.
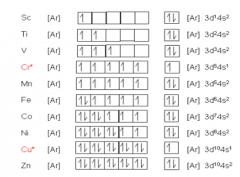
11 Dec 2019 — draw the orbital diagram for the ion co2+. Answer. +20. Watch. 1. answer. 0. watching. 258. views. For unlimited access to Homework Help, ...1 answer · Top answer: Draw the orbital diagram for the ion . Neutral cobalt has electrons with an electron configuration of Notice here that is written after . This is because ...

Co2+ orbital diagram
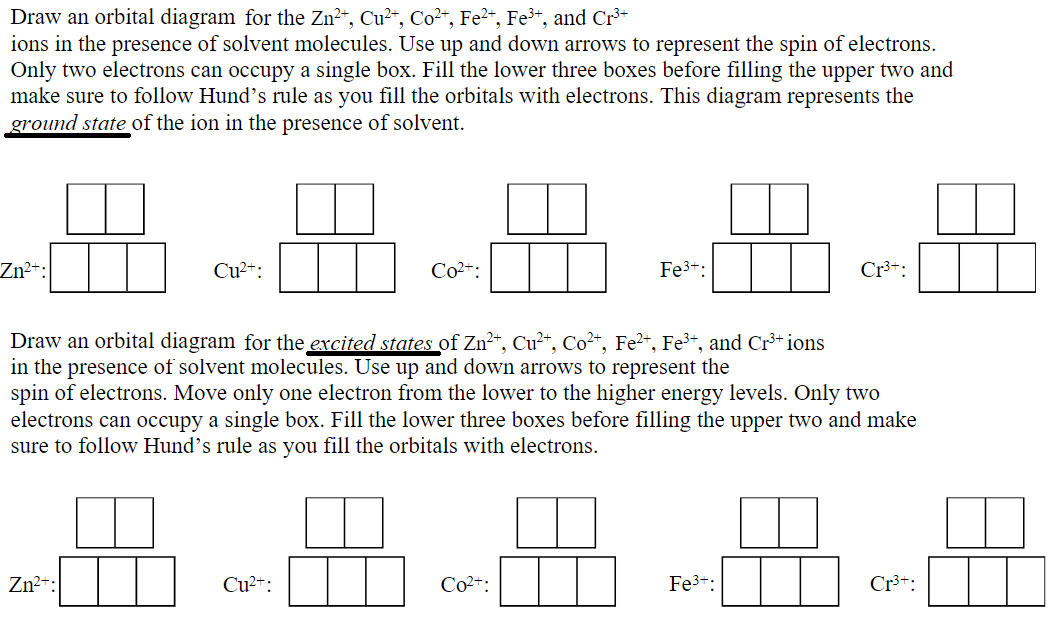
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons. Part C. Draw the orbital diagram for the ion N3−. ... this new set of axes, the orbitals on Co 2+ can be labelled and the ground state can be seen in a single Slater's determinant approximation, as ... Carbon dioxide has a formal double bond between C-O. ... The molecular orbital diagram of carbon monoxide is very similar to that of molecular nitrogen. Carbon, with 4 valence electrons, and oxygen with 6 valence electrons, together have the same number of electrons as dinitrogen.
Co2+ orbital diagram. Carbon dioxide (CO2), molecule is triatomic and linear like Beryllium di hydride (BeH2) However, unlike hydrogen as peripheral atoms in BeH2, there are oxyge... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. 28 Aug 2020 — Use an orbital diagram to describe the electron configuration of the valence shell of each of the following atoms:. 6.2.2: Carbon dioxide. Construct SALCs and the molecular orbital diagram for CO 2. Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principle axis. Step 2. Identify and count the pendant atoms' valence orbitals. Step 3. Generate the Γ 's. Step 4.
Watch the video solution for the question: Draw the orbital diagram for ion Co 2+.. . can be accommodated in the metal d orbitals. • d0 ions •d7 ions - Fe1+, Ru1+, Co2+, Rh2+, Ni3+, etc. . σ-ML4 Tetrahedral MO Diagram e. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbitals. Apr 30, 2017 · Bond length increases from left to right on your list, i.e. r_(CO) < r_(CO^(+)) < [r_(CO^(2+)) = r_(CO_2)] < r_(CO_3^(2-)) "CO": 3 "CO"^(+): 2.5 "CO"^(2+): 2 "CO"_2: 2 "CO"_3^(2-): 1.bar(33) In order to determine this, we should reference an MO diagram. We can see that the highest occupied molecular orbital (HOMO) is fully occupied, but the next-highest MOs are the pi^"*" antibonding lowest ... The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is #2# , each with opposite spins (Pauli's exclusion principle). In a neutral carbon atom, the #"1s"# sublevel has one orbital with two electrons with opposite spins, represented by the arrows pointing in opposite ... 40 draw the orbital diagram for the ion co2+. Written By Kathy W. Blatt. Wednesday, November 17, 2021 Add Comment Edit. Answer (1 of 3): The atomic orbital s of oxygen are uni for mly lower in energy than the corresponding atomic orbital s of element C because of the increased stability of the electrons in oxygen.
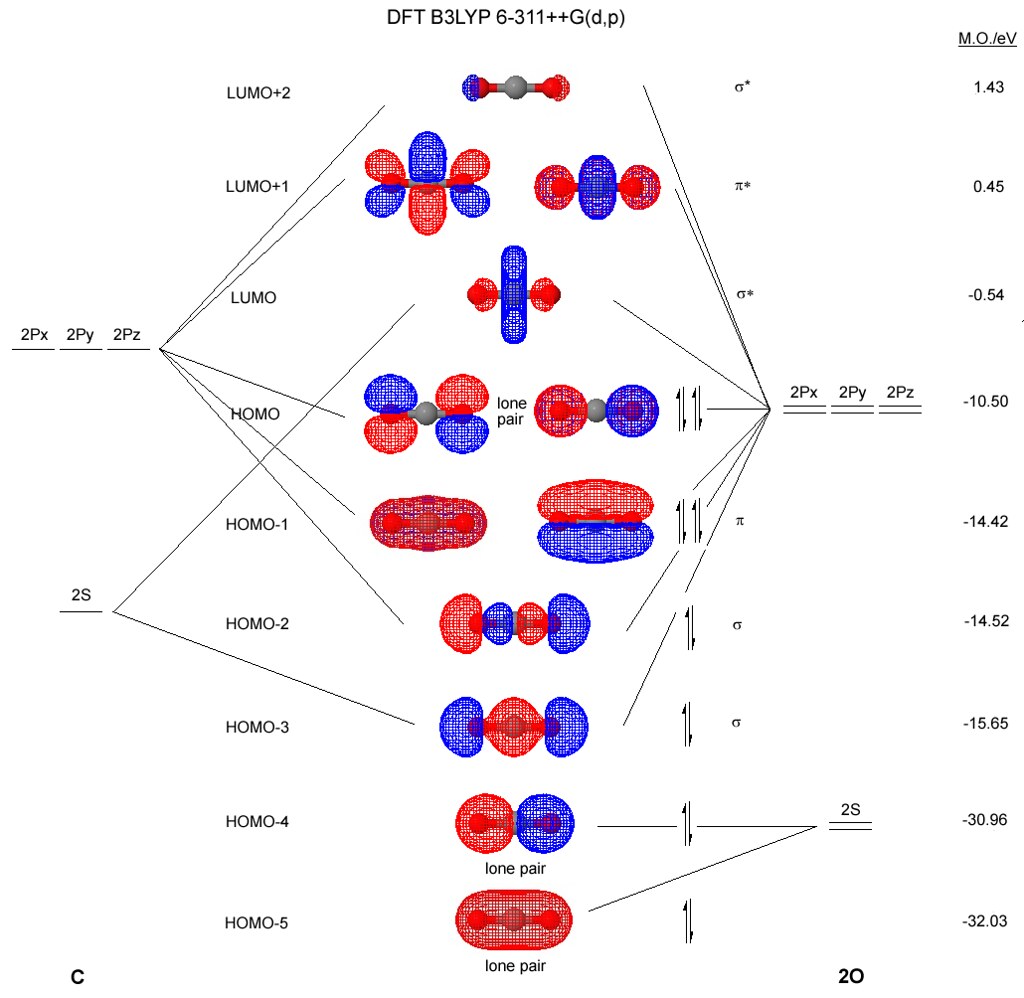
File:MO Diagram CO2.svg. Size of this PNG preview of this SVG file: 406 × 599 pixels. Other resolutions: 162 × 240 pixels | 325 × 480 pixels | 406 × 600 pixels | 520 × 768 pixels | 694 × 1,024 pixels | 1,387 × 2,048 pixels | 420 × 620 pixels. Nov 11, 2021 · Now the next topic to cover is the molecular orbital diagram of nitrous oxide. N2O Molecular Orbital Diagram. Molecular orbital diagrams say about the mixing of orbitals in a compound. Using a MO diagram, the bond order of a compound can be determined which gives us an idea about bond length, bond stability as well. Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at. Ni2+ Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following. a. Zn2+ b. Co2+ (high and low spin) c. Ti3+ the FT ligand. An orbital diagram, or orbital filling diagram, is a type of notation which ... Next, the video goes over an ions with a charges S2−, Al3+, and Co2+.
Carbon Dioxide by Reducible Representations Γ2s= Ag+ B1u Γ2pz= Ag+ B1u Γ2px= B2g+ B3u Γ2py= B3g+ B2u B3u B2u Ag Ag 2p x 2p y 2p z 2s B2g B3g B1u B1u These are the same group orbital symmetries that we got using inspection. We can (re)draw them. 5. Find matching orbitals on central atom Ag B1u B3u B2u 6. Build MO diagram…
The energy diagram for carbon in CO 2 is shown below. What is the hybridization of oxygen in CO 2. Each oxygen has two lone pairs and forms one s bond and one p bond. This means that there must be three hybridized orbitals and one unhybridized p orbital to make the p bond. This is sp 2 hybridization.

93 Sun Ired Electrons Present In The Orbital Sum Of The Paired Electrons Present In Wil 2 In All The Species Fe2 Co2 And Ni 2 Are 1 9 2 12 3 6 4 15
Aug 15, 2020 · Diagram of Stretching and Bending Modes for H 2 O. Solution. H 2 O molecule is a non-linear molecule due to the uneven distribution of the electron density. O 2 is more electronegative than H 2 and carries a negative charge, while H has a partial positive charge.
Solved Build A Galvanic Cell From An Au3 Au And Co3 Co2 Couple The Salt Bridge Solution Contains Kci Au3 Aq 3e Au S E 1 50 V Co3 A Course Hero
Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry ...
26 Jan 2021 — The Cobalt Electron Configuration (Co) with Orbital Diagram and Cobalt valence electrons have been provided here with the pictures.

Aqueous Solutions Of Co2 Salts Are Pale Pink They Contain The Complex Ion Co H2o 6 2 Addition Of Homeworklib
Molecular Orbitals for CO2. Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on an orbital in the energy level correlation diagram shown Mouse Control of Models. Left mouse drag rotate; Shift Left drag resize; Shift Right drag z-rotate; Right click for menu Notes
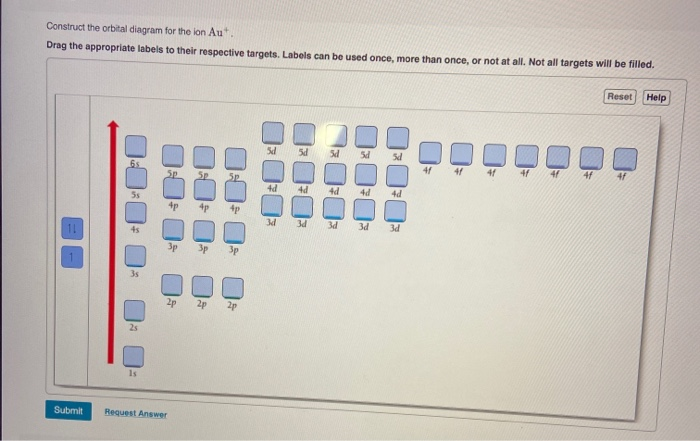
Q. Write orbital diagram for the followings:a. Au+b. Zr2+. Q. Draw the orbital diagram for ion Ca 2+. Q. Draw the orbital diagram for ion N 3-. Q. Draw the orbital diagrams (box/line notation) for the following species:Mn2+Cu. See all problems in The Electron Configuration: Ions.
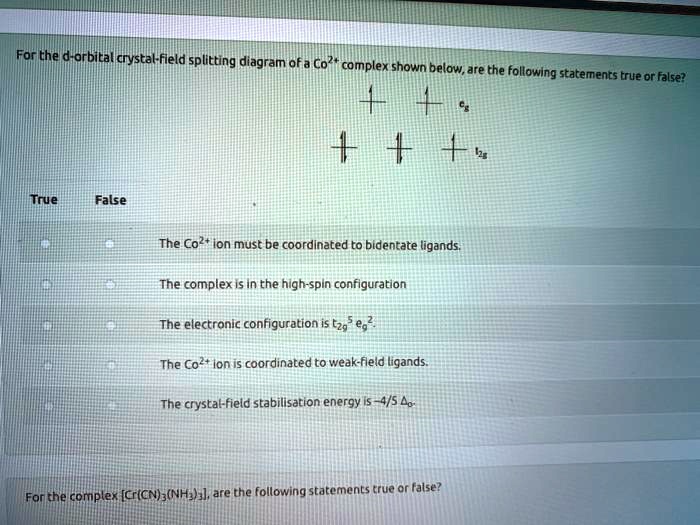
Solved Forthe D Orbital Crystal Field Splitting Diagram Of A Co2 Complex Shown Below Are The Following Statements True Or False True False The Co Ion Must Be Coordlnated To Bidentate Ligands The Complex Is In
Why does Co2+ have 7 electrons in the 3d orbital, and not 5 like Mn? Ask Question Asked 7 years, 3 months ago. Active 3 years, 3 months ago. ... But while you fill $3d$ orbital with electrons it becomes lower and lower in energy and eventually ends up lower in energy than the $4s$ orbital. Thus, when electrons are lost from $\ce{Co}$ atom, they ...
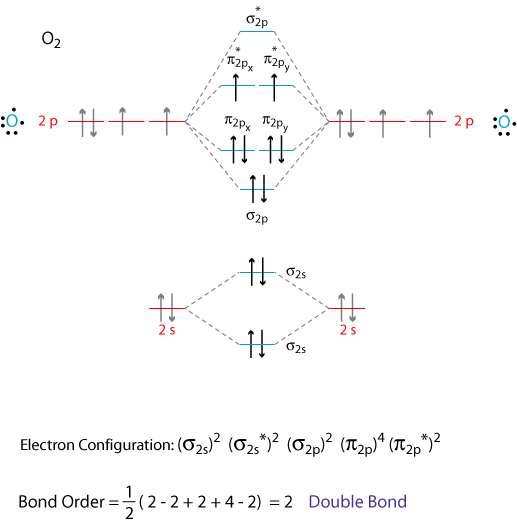
The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.
Davidson
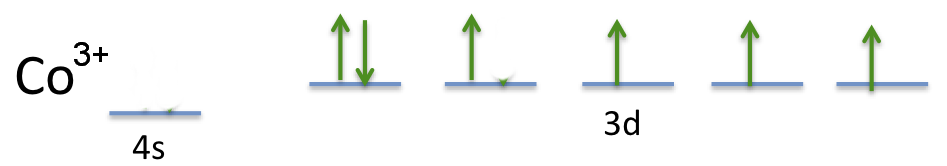
How Many Unpaired Electrons Are In A Gaseous Co 3 Ion In Its Ground State The Answer Is 4 But I Don T Understand How Could Someone Please Explain Thank You Socratic
Answer (1 of 3): The atomic orbitals of oxygen are uniformly lower in energy than the corresponding atomic orbitals of element C because of the increased stability of the electrons in oxygen. The molecular orbitals are no longer symmetrical, and the energies of the bonding molecular orbitals are ...

Two Orbital Three Electron Stabilizing Interaction For Direct Co2 As3 Bonds Involving Square Planar Coo4 In Bacoas2o5 David 2014 Angewandte Chemie Wiley Online Library
The Penultimate Glacial Period (PGP) is the glacial period that occurred before the Last Glacial Period.It began about 194,000 years ago and ended 135,000 years ago, with the beginning of the Eemian interglacial.
Oct 24, 2018 · diagram for CO2 in Figure can be used as a guide, with the orbitals of Be higher in energy than those of C and the orbitals of F lower in energy than those of O. Calculated molecular orbital shapes are below, for comparison for those of CO 2 in Figure The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic ...

Scielo Brasil Study Of Co2 In Different Crystal Field Environments Study Of Co2 In Different Crystal Field Environments
Chapter 5 / Lesson 16. 16K. The bonds between atoms in molecules have different shapes, sizes, and strengths depending on which atoms are bonded together. Learn how to apply molecular orbital ...
Nov 08, 2021 · The molecular orbital diagram of SO2 is attached below: A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital. This further helps us to find out the bond order, bond length, and bond strength of any compound.
5.4.2 Carbon Dioxide's Molecular Orbital Diagram O=C=O D∞h-> D 2h 5.4 Molecular Orbitals for Larger Molecules 5.4.2 Carbon Dioxide's Molecular Orbital Diagram O=C=O D∞h-> D 2h Oxygen Group Theory Carbon Group Theory
Fig. 7: diagram showing how the electrons fill based on the Aufbau principle. The π bonding orbital is lower in energy than the nonbonding p orbital. Since every carbon center shown has two electrons in the lower energy, bonding π orbitals, the energy of each system is lowered overall (and thus more stable), regardless of cation, radical, or ...
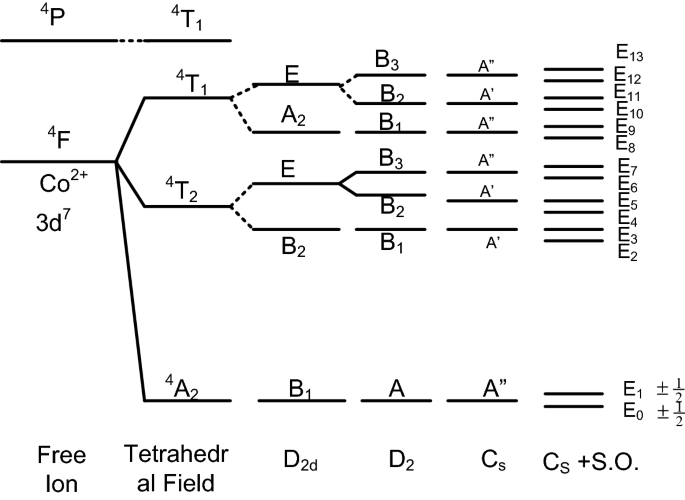
Origin Of The Ground Kramers Doublets For Co2 3d7 Ions With The Effective Spin 3 2 Versus The Fictitious Spin Springerlink
I am fairly sure the first diagram I drew for carbon dioxide is wrong in terms of showing π bonding. This is because we use a π orbital twice, which isn't possible. The second diagram corrects this by realizing there are two unused p orbitals on the carbon.
In carbon dioxide molecule, oxygen also hybridizes its orbitals to form three sp 2 hybrid orbitals. The p orbital in oxygen remains unchanged and is mainly used to form a pi bond. However, out of the three sp hybrid orbitals, only one will be used to form a bond with the carbon atom.
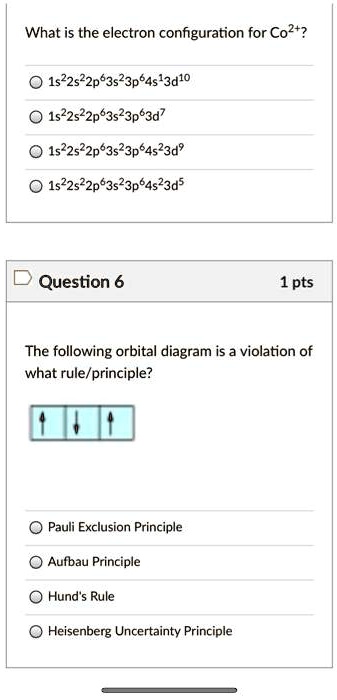
Solved What Is The Electron Configuration For Co2 1s22522p63523p64s 3d10 1522522p63523p83d7 1522522p63523p84523d9 1s22s22p63s23p84s23d5 Question 6 1 Pts The Following Orbital Diagram Is A Violation Of What Rule Principle Pauli Exclusion Principle
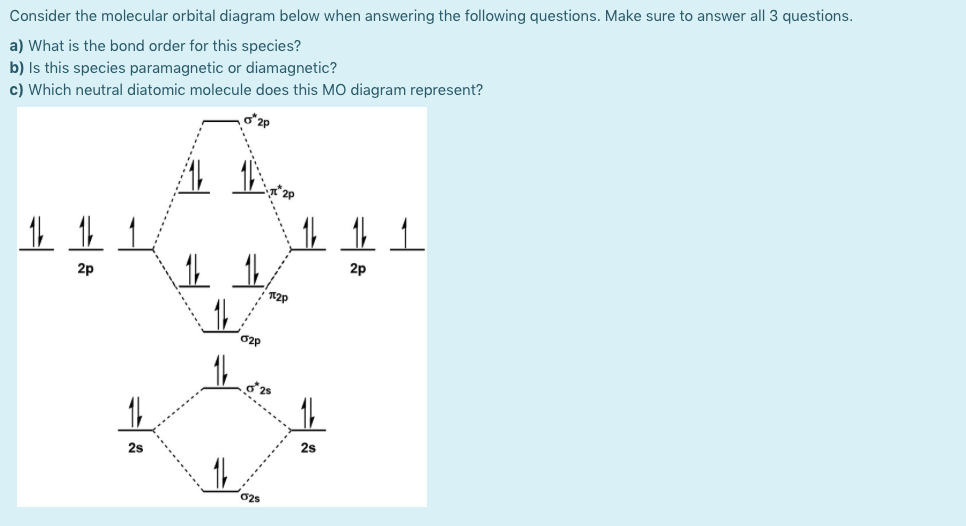
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
ISBN-13: 9781938168390 ISBN: 1938168399 Authors: Richard Langley, Klaus Theopold, William R. Robinson, Paul Flowers Rent | Buy. Alternate ISBN: 9781630181833. Alternate ISBN: 9781630181833. Chemistry (0th Edition) Edit edition Solutions for Chapter 8 Problem 31E: Multiple Bonds Draw the orbital diagram for carbon in CO2 showing how many carbon ...
A description of the hybridization of CO2 including sigma and pi bonds.Note that the CO2 hybridization is sp for the central carbon atom. It's sp2 for each ...
Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add.May 09, · This feature is not available right now.
Carbon dioxide has a formal double bond between C-O. ... The molecular orbital diagram of carbon monoxide is very similar to that of molecular nitrogen. Carbon, with 4 valence electrons, and oxygen with 6 valence electrons, together have the same number of electrons as dinitrogen.
... this new set of axes, the orbitals on Co 2+ can be labelled and the ground state can be seen in a single Slater's determinant approximation, as ...
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals. Click within an orbital to add electrons. Part C. Draw the orbital diagram for the ion N3−.

Figure 1 2 From The Chemistry Of Tris Phosphino Borate Manganese And Iron Platforms Semantic Scholar














0 Response to "42 co2+ orbital diagram"
Post a Comment