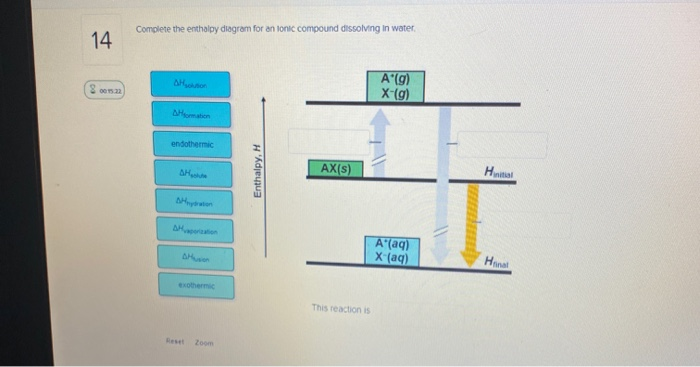
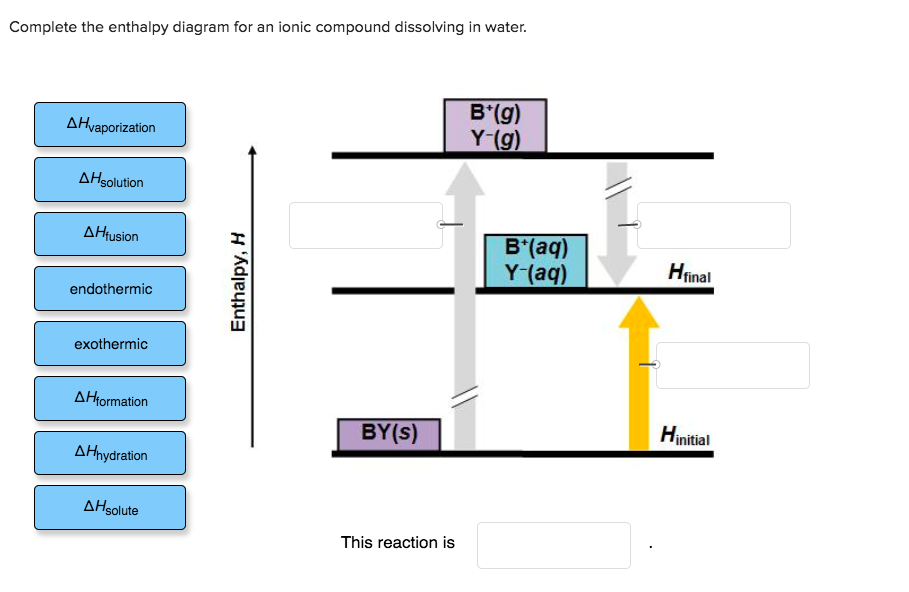
42 complete the enthalpy diagram for an ionic compound dissolving in water.
FREE Answer to Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y-(g) vaporization Δ... Ionic compounds dissolved in water switch partners. One compound breaks into elements or smaller compounds. Two or more elements or compounds combine to form one product. Part of an ionic compound is removed and replaced by a new element. 5. Define the following terms as they are commonly used in the English language. Synthesis Decomposition
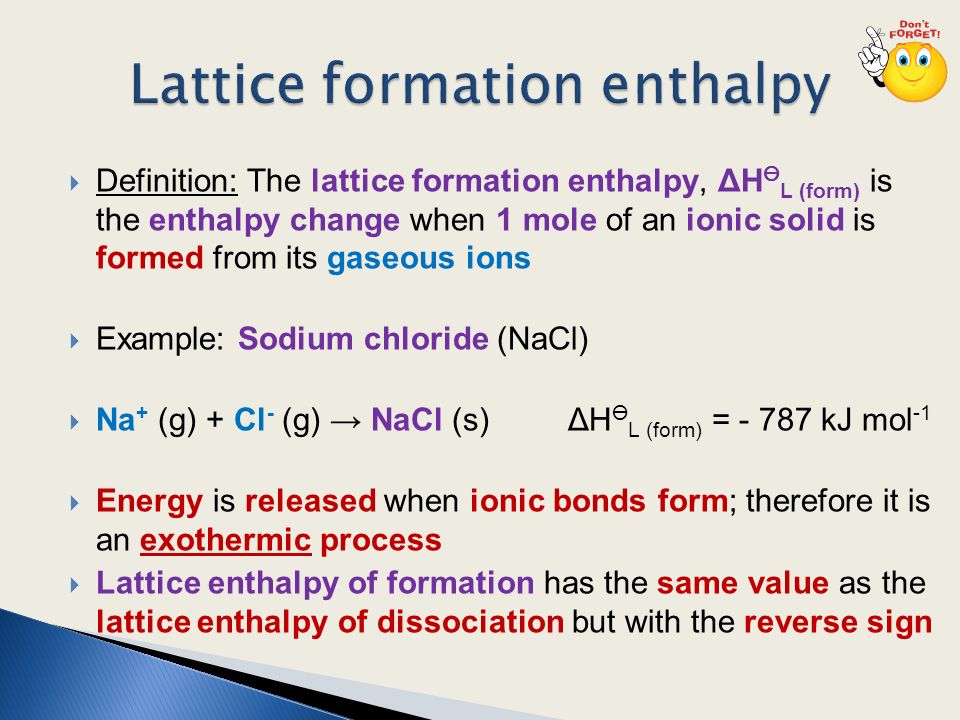
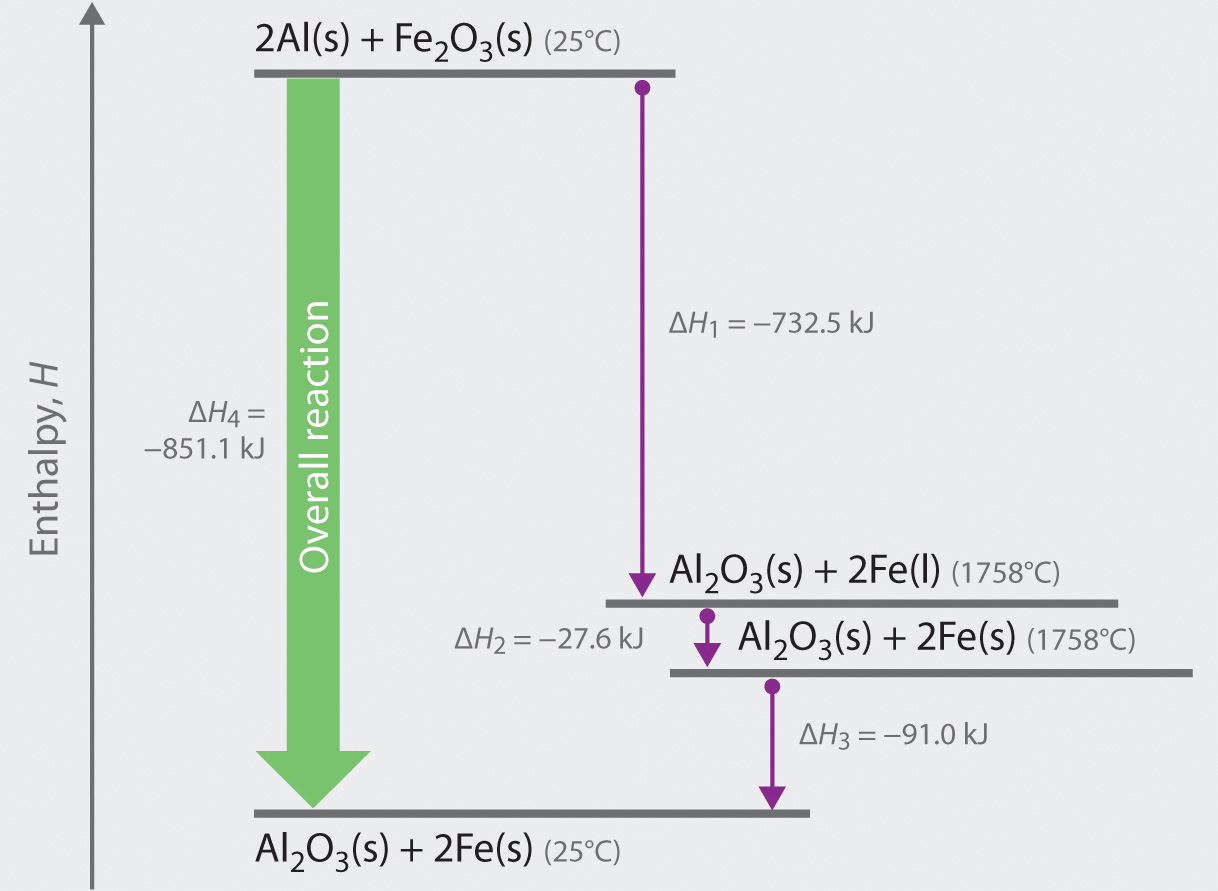
When an ionic substance dissolves in water, its enthalpy of solution depends on the difference between its lattice enthalpy and the enthalpies of hydration of its ions. The enthalpy of solution can be calculated with the help of an enthalpy cycle diagram. Calculating the enthalpy of solution,
Complete the enthalpy diagram for an ionic compound dissolving in water.
Some ionic compounds give off heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives off or absorbs heat depends on the strength of the intermolecular forces holding the solid together, as well as those between the ions and the water once dissolved. Enthalpy change of solution and Enthalpy change of Hydration When ionic compounds dissolve in water, there is usually a temperature change. Sometimes this is exothermic (e.g. dissolving calcium chloride) and sometimes endothermic (e.g. dissolving ammonium nitrate). The experiments are easy to carry out in a laboratory, and the usual equations ... Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y (g) AHsolution B' (aq) Y- (aq) Ahydration Ht final exothermic H solute AHvaporization BY (S) Hinitial endothermic Hormation This reaction is.
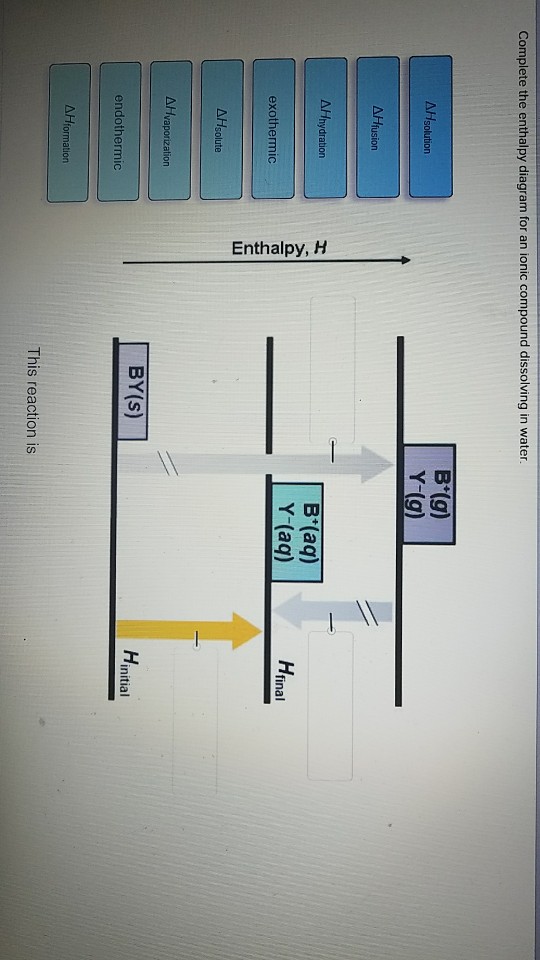
Complete the enthalpy diagram for an ionic compound dissolving in water.. 4 *P44255A0420* 2 The solubility of a solid in water is the maximum mass of the solid that can dissolve in 100 g of water at a given temperature. An aqueous solution containing this maximum mass can be described as a saturated solution. The graph shows the solubilities of three solids at different temperatures. Transcribed image text: Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y-(g) vaporization Δ Hsolution B(aq) Y (aq) fusion ... Some ionic compounds dissolve readily in water, while others are insoluble. Some ionic com-pounds give o heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives o or absorbs heat depends on the strength of the inter-molecular forces holding the solid together, as well as those ... dissolving hydrated salts in water ... Image: Enthalpy diagram ... enthalpy change that occurs when one mole of a solid ionic compound is separated into ...
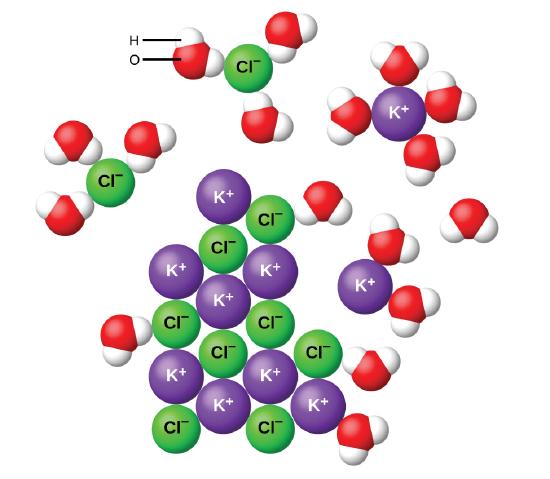
Ionic compounds are often soluble in water, because the attractions formed between ions and water are frequently strong enough to make their solution either exothermic or only slightly endothermic. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Dissociation. An ionic crystal lattice breaks apart when it is dissolved in water. Dissociation is the separation of ions that occurs when a solid ionic compound dissolves. It is important to be able to write dissociation equations. Simply undo the crisscross method that you learned when writing chemical formulas of ionic compounds. Many ionic compounds are soluble in water, however, not all ionic compounds are soluble. Ionic compounds that are soluble in water exist in their ionic state within the solution. You will notice in Figure 7.2 that the sodium chloride breaks apart into the sodium ion and the chloride ion as it dissolves and interacts with the water molecules. a. Water, H 2O ionic compound covalent compound b. Sodium chloride, NaCl ionic compound covalent compound c. Calcium carbonate, CaCO 3 ionic compound covalent compound d. Hydrogen chloride, HCl ionic compound covalent compound e. Glycerol, C 3H 8O 3 ionic compound covalent compound 3. Nitrate is a polyatomic ion with a charge of -1.
Dissolving an ionic salt in water Computer Simulation and Computer Animation A short computer animation illustration how positive and negative ions in a solid ionic compound dissolve in water might be used to accompany dissolving salts in water demonstration. The solution (including the reactants and the products) and the calorimeter itself do not undergo a physical or chemical change, so we need to use the expression for specific heat capacity to relate their change in temperature to the amount of heat (q cal) that they have exchanged (Eqn. 3). In Eqn. 3, m is the mass (mass of the reactants + mass of water + mass of calorimeter), C is the ... the enthalpy ch an ge when one mole of an ionic compound dissolves in sufficient water to produce a solution of in f in ite dilution. Enthalpy ch an ges c an be determ in ed in the laboratory us in g ... Get 24⁄7 customer support help when you place a homework help service order with us. Ionic compounds dissolve in water because the water molecules hydrate the ions. > To dissolve an ionic compound, the water molecules must be able to stabilize the ions that result from breaking the ionic bond. They do this by hydrating the ions. Water is a polar molecule. It has a permanent dipole. The "O" atom has a partial negative charge, and the "H" atoms have a partial positive charge.
The enthalpy change of solution is the enthalpy change when 1 mole of an ionic substance dissolves in water to give a solution of infinite dilution. Enthalpies of solution may be either positive or negative - in other words, some ionic substances dissolved endothermically (for example, NaCl); others dissolve exothermically (for example NaOH).
When water dissolves a substance, the water molecules attract and "bond" to the particles (molecules or ions) of the substance causing the particles to separate from each other. The "bond" that a water molecule makes is not a covalent or ionic bond. It is a strong attraction caused by water's polarity.

2 0 Calorimetry Enthalpy Of Solution Lab 2020 Docx Sch4u0 U2013 Calorimetry Lab Enthalpy Of Solution U2206hsoln Of Ionic Compounds Introduction The Course Hero
A common example of this type of ionic compound is sodium chloride, commonly known as table salt. As noted at the beginning of this module, spontaneous solution formation is favored, but not guaranteed, by exothermic dissolution processes. While many soluble compounds do, indeed, dissolve with the release of heat, some dissolve endothermically.
Dec 11, 2019 — Get the detailed answer: Complete the enthalpy diagram for an ionic compound dissolving in water.
1 answerThe strength of the intermolecular forces keeping the solid connected, as well as those between the ions and the water once dissolved, govern if the ...
Calculate the amount of heat required (in kilojoules) to heat 5.00 grams of water from -16.0 C to 11.0 c.-enthalpy of vaporization for water is 40.56 kJ/mol-enthalpy for fusion of water is 6.007 kJ/mol -specific heat for ice is 2.090 J/(gram x *C)-specific heat for water is 4.184 J/(gram x *C)-specific heat for steam is 2.030 J/(gram x *C)
2S, is an ionic compound of sodium, Na, and sulfur, S. (a) Draw a 'dot-and-cross' diagram to show the bonding in sodium sulfide. Show outer electrons only. [2] (b) The table below compares the properties of sodium sulfide, sodium and sulfur. Complete the table. Sodium sulfide Sodium Sulfur Melting point / °C 1180 98 113 Type of structure
1 (a) The dissolving of an ionic compound in water is accompanied by an energy change, the enthalpy change of solution, ∆H sol. MgCl 2+2(s) + aq → Mg (aq) + 2Cl -(aq) Describe, in terms of bond breaking and bond making, what happens to the solid ionic lattice when an ionic compound dissolves in water.

Complete The Enthalpy Diagram For An Ionic Compound Dissolving In Water B G Y G Vaporization D Homeworklib
We can generally assume that salts dissociate into their ions when they dissolve in water. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution.

Complete The Enthalpy Diagram For An Ionic Compound Dissolving In Water B G Y G Vaporization D Homeworklib
Terms in this set (70) Combustion Reactions. A reaction in which a substance reacts with oxygen, emitting heat and forming one or more oxygen-containing compounds. Evidence of a chemical reaction: -color change. -formation of a solid in a previously clear solution. -the formation of a gas when we add a substance to a solution.
complete the enthalpy diagram for an ionic compound dissolving in water. Answer + 20. Watch. 1. answer. 0.
Chemistry. Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water. A (g) X- (g) AHnydration AHusion exothermic AX (s) initial ??,aporization endothermic A (aq) x- (aq) A Hsolution Hrinal ??,ormation AHsolute This reaction is.
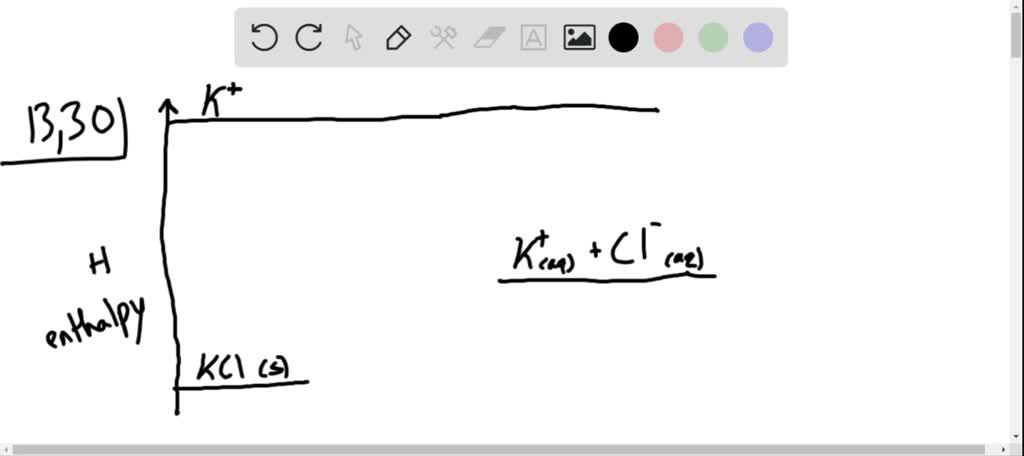
Solved Complete The Enthalpy Diagram For An Ionic Compound Dissolving In Water Exothermic B7g Y G B Aq Y Laq Endothermic Ahvaporzation Lhspute Ahnydration Hiinal Ahiusion Ahformation By S Hinitial Ahsolution This Reaction Is
Big idea water is polar with regions of slight positive and negative charge. Acs general chemistry final study guide. The lattice energy must be supplied in ...
4 answersComplete the enthalpy diagram for an ionic compound dissolving in water: exothermic B7g) Y-(g) B(aq) Y-laq) endothermic AHvaporzation LHspute ] AHnydration ...
The Dissolving Process. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in water. We will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. Water molecules move about continuously due to ...
Complete the enthalpy diagram for an ionic compound dissolving in water b g y g ahsolution b. This enthalpy of solution δhsolution can either be positive endothermic or negative exothermic. With the aid of a diagram show the enthalpy changes involved in the dissolving of an ionic compound in water where enthalpy change of solution is exothermic.
When An Ionic Solid Dissolves In Water Two Processes Occur Firstly The Ions Are Separated Endothermic Secondly The Ions Are Surrounded By Water Ppt Download
(ii) The temperature change in this experiment shows that dissolving lithium iodide in water to form lithium iodide solution is an exothermic process. Complete the energy level diagram to show the position of the lithium iodide solution. Label the diagram to show ûH, the molar enthalpy change. (2) (Total for Question 5 = 11 marks)
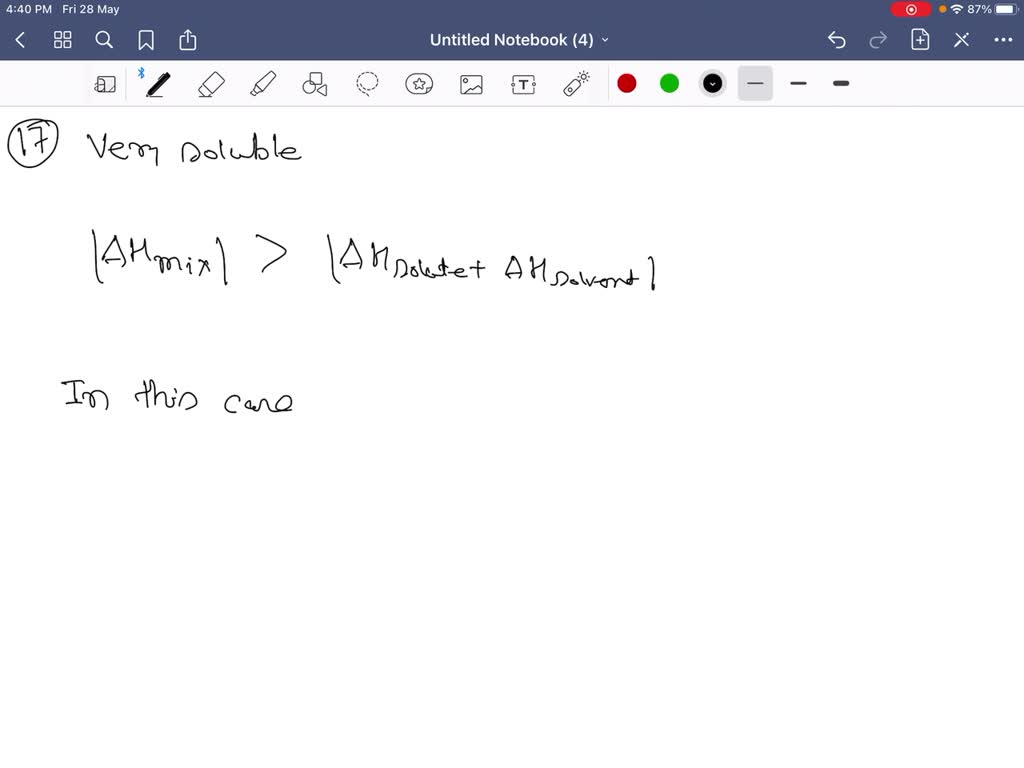
Solved 10 Points When An Ionic Compound M X Is Added To Water The Temperature Of The Water Increases Draw Jn Enthalpy Diagram That Shows The Solution Formation As Step Process Extru Credit
Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y-(g) vaporization Δ Hsolution B(aq) Y (aq) fusion final endothermic ...

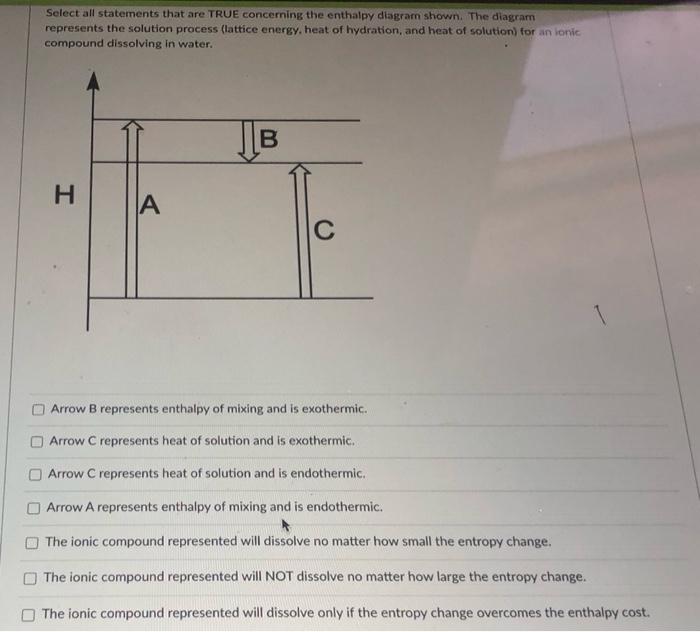
Please Help Me Find All The True Select All Statements That Are True Concerning The Enthalpy Homeworklib
A complete enthalpy diagram will include ... An example of this is when we dissolve ammonium chloride in water. If you do this in a test tube you can actually feel the test tube getting colder ...
Chemistry questions and answers. Complete the enthalpy diagram for an ionic compound dissolving in water B (g) Y (g) AHsolution B' (aq) Y- (aq) Ahydration Ht final exothermic H solute AHvaporization BY (S) Hinitial endothermic Hormation This reaction is.
Enthalpy change of solution and Enthalpy change of Hydration When ionic compounds dissolve in water, there is usually a temperature change. Sometimes this is exothermic (e.g. dissolving calcium chloride) and sometimes endothermic (e.g. dissolving ammonium nitrate). The experiments are easy to carry out in a laboratory, and the usual equations ...
Some ionic compounds give off heat when dissolving in water and others absorb heat. Whether the dissolution process of a given ionic compound gives off or absorbs heat depends on the strength of the intermolecular forces holding the solid together, as well as those between the ions and the water once dissolved.

When An Ionic Solid Dissolves In Water Two Processes Occur Firstly The Ions Are Separated Endothermic Secondly The Ions Are Surrounded By Water Ppt Download
Thermal Properties Crystal Structures And Phase Diagrams Of Ionic Plastic Crystals And Ionic Liquids Containing A Chiral Cationic Sandwich Complex Physical Chemistry Chemical Physics Rsc Publishing
Energies Free Full Text Sub And Supercritical Water Liquefaction Of Kraft Lignin And Black Liquor Derived Lignin Html

Please Help Me Find All The True Select All Statements That Are True Concerning The Enthalpy Homeworklib
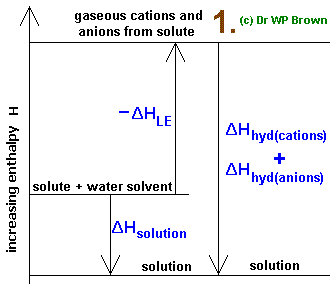
I Have Read That When Metal Like Na K Be Put Into Water Heat Is Evolved Can We Call This Heat Of Hydration Please Let Me Know What Is Heat Of

Enthalpy Of Reaction And Calorimetry Worksheet H Rdquo H An Enthalpy Diagram Showing The Enthalpy Changes
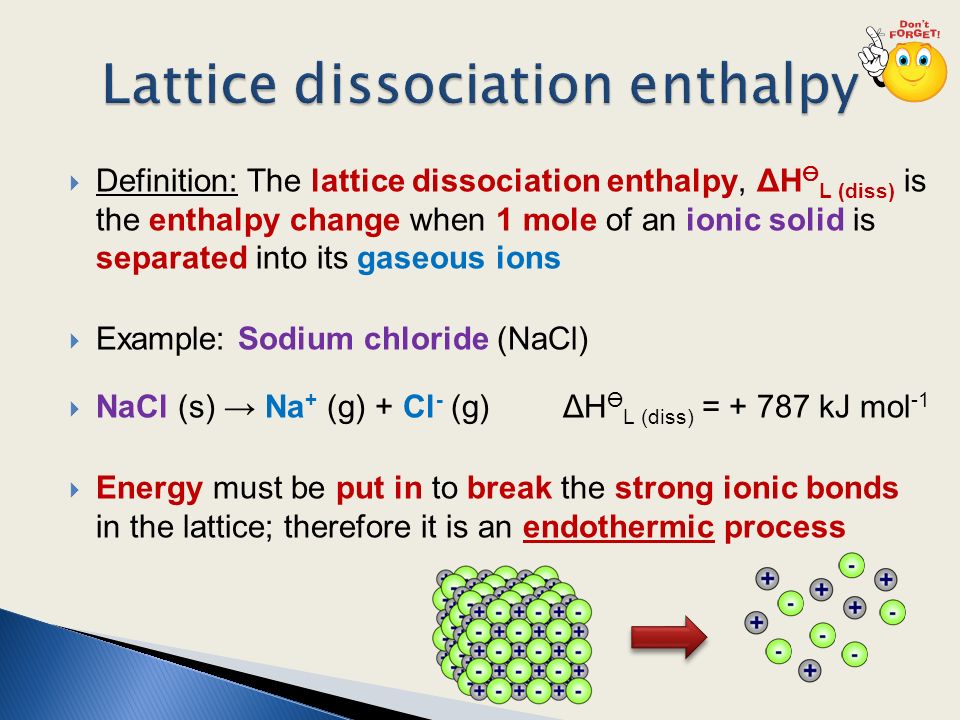
When An Ionic Solid Dissolves In Water Two Processes Occur Firstly The Ions Are Separated Endothermic Secondly The Ions Are Surrounded By Water Ppt Download
Processes Free Full Text On The Application Of The Factsage Thermochemical Software And Databases In Materials Science And Pyrometallurgy Html








0 Response to "42 complete the enthalpy diagram for an ionic compound dissolving in water."
Post a Comment