44 lewis diagram for so3
So 3 is named sulfur trioxide. So 6 minus zero minus 12 over 2. Diagram Of So3 Wiring Diagram The lewis dot stru... How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check...
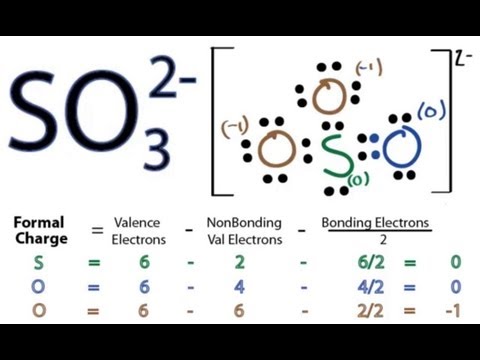
Lewis structure of sulfite ion is drawn in this tutorial. Total valence electrons concept is used to draw the lewis structure. For three oxygen atoms, nine electrons pairs are spent. Now one electron pair (10-9) is remaining. Mark remaining electron pair on sulfur atom.
Lewis diagram for so3
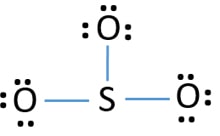
Lewis structure of SO3. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation. SO₃ stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes. To draw the lewis structure for SO2, we have to find out the valence electrons of sulfur and oxygen first.We express valence electrons as dots in lewis dot structure.
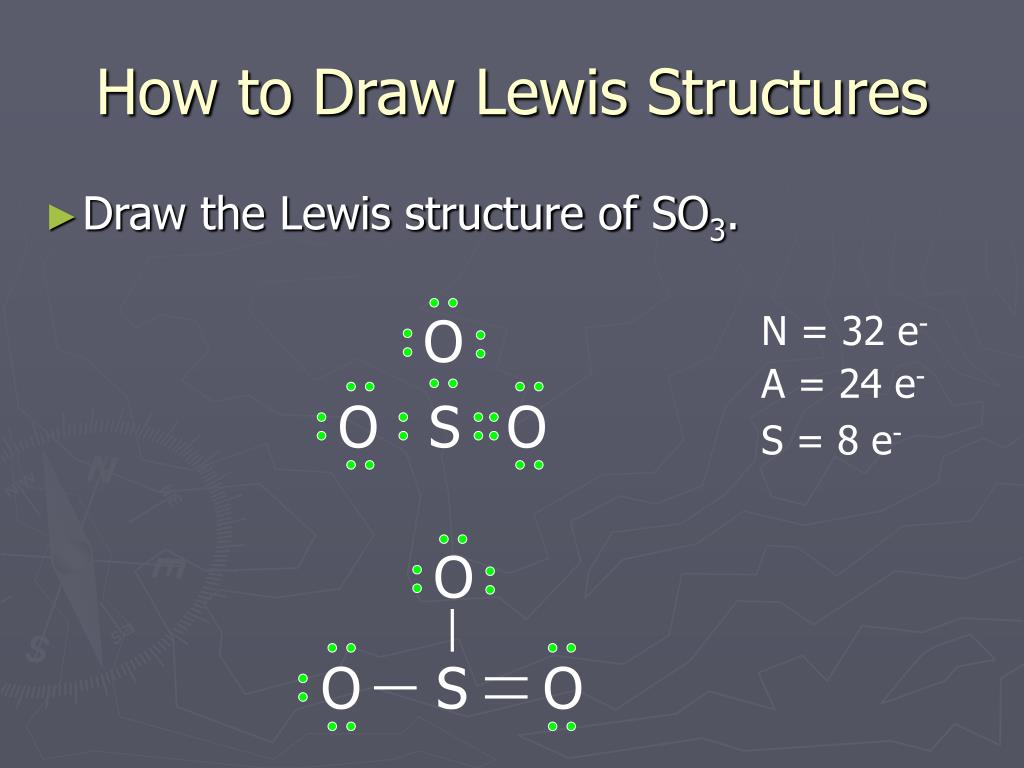
Lewis diagram for so3. In the SO3 Lewis structure diagram, the sulfur atom can be the center atom of the molecule. As a result, central sulfur in the SO3 Lewis structure, with all three oxygen atoms arranged in trigonal planar geometry. Add valence electrons around the oxygen atom, as given in the figure. The geometry of SO3 is trigonal planar with a symmetric charge distribution. Therefore this molecule is nonpolar. Problem3 Feedback This is an example of an expanded octet. Problem2 To answer the questions, interpret the following Lewis diagram for SO2. Lewis dot structure of the sulfite ion so 3 2 a simple method for writing lewis structures is given in a previous article entitled lewis st... Lewis Dot Diagram For So3 - Wiring Diagram. What are all resonance structures for SO3? | Socratic. SO3 Lewis Structure, Molecular Geometry, and Hybridization ... What is the difference between the Lewis structure of SO3 ... Chemistry Class 11 NCERT Solutions: Chapter 4 Chemical ...
For example, the Lewis electron dot diagram for calcium is simply. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent... Lewis Structures or electron dot diagrams for atoms, ions, ionic compounds and covalent compounds tutorial with worked examples for chemistry students. So, if we let X represent the symbol of an atom of an element, we can write a general Lewis structure for each of the main group elements Total of 24 electrons used. It will hold more than 8 electrons. Lewis Dot Structure So3 2 Lewis dot of sulfur tr... Drawing Lewis dot structures (also known as Lewis structures or Lewis diagrams) can be confusing, particularly for a beginning chemistry student. Therefore, 2 of the electrons are required to make a double bond between the atoms so the octet rule is met for both.
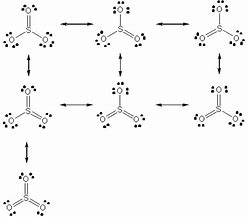
So far we have introduced Lewis diagrams accord-ing to a few simple rules, which are sufficient to write the diagrams of the simple molecules we have been For each of the molecules shown on pages 12-14 draw the Lewis/line diagram. Another molecule which exhibits resonance is SO3. Lewis Symbols. Electron Configuration into Shells. The first shell (n=1) can have only 2 electrons, so that shell is filled in helium, the first noble gas. In the periodic table, the elements are placed in "periods" and arranged left to right in the order of filling of electrons in the outer shell. THE MO DIAGRAM FOR SO3 Here is the MO diagram for "SO"_3: To account for the electrons, we have: The 3a_1' and In the context of Organic Chemistry, a Lewis acid is also called an electrophile, or a lover of electrons. # "SO"_3# exhibits electrophilic behavior when a nucleophile (electron donor)... Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
Solved Draw Lewis Structure For The Following So2 Ph3 Ch2cl2 So3 2 C2f2 Cs2 Indicate If The Molecules Are Polar Or Non Polar So2 Ph3 Ch2cl2 So3 2 Course Hero
SO3 which is also spelled as Sulphur Trioxide sometimes, is a trigonal planar molecule that is non-flammable. In this article, I will provide you some information regarding SO3 molecular geometry with the explanations of Lewis structure, polarity, and hybridization.
Lewis Diagram for SO3. S.j Bahula. This video describes "Lewis Dot Structure of SO3,SO2,CrO5,H2SO5,H2S2O8 and HNO4" It helps students to develop depth concepts in chemistry who are ...
The Lewis structure for SO 3 is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 3 at first. You'll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for SO 3 . SO 3 is a good...
A Lewis Dot Structure is drawn by a series of dots, lines, and atomic symbols and provides a structure for the way that the atom or molecule is arranged. A Lewis Dot Structure can be made for a single atom, a covalent compound, or a polyatomic ion.
› Get more: So3 lewis structure molecular geometryShow All. Lewis Dot Structure of SO3 (Sulfur Trioxide). How. Details: Drawing the Lewis Structure for SO 3. Viewing Notes: The Lewis structure for SO 3 is requires you to place more than 8 valence electrons on Sulfur (S).; You might think you've...
rules for trying to draw these Lewis diagrams so the first example that we will look at is silicon tetrafluoride and tetrafluoride is just a fancy way of saying four fluorines so tetra fluoride now the first step is to say well what are the electrons that are of interest to us and if we're talking about the...
There are pair(s) around the central atom, so the geometry of SO_3 is.
Chapter 4 Part 2 Lewis Structures. U30105 Steps U3011lewis Dot Structure How To Do. Chemistry - Lewis Dot Diagrams. Bohr-rutherford Diagrams U0026 Lewis Dot Diagrams. Ps3. Lewis Diagram U2013 Charts. Worksheet Electron Dot Diagrams And Lewis Structures.
IMPORTANT : no Lewis diagram is complete without formal charges. Lewis diagrams are drawn to examine mechanisms so knowing which parts of a molecule are electron defficient (+) and which are electron rich (-) is vital. It is best to have a formal charge of 0 for as many of the atoms in a structure...
How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanation Sulfur is an exception to the octet rule - it can handle up ... Ketzbook demonstrates how to draw Lewis diagrams for elements and simple molecules using an easy to follow step-by-step ...
Lewis Structure of SO3 Valence: Here, sulfur in the center because of its lowest electron capability, and three oxygen around it. A Lewis structure for SO3 that obeys the octet rule, showing all non-zero formal charges, is shown he many resonance structures for SO3 that obey the octet rule, are possible...
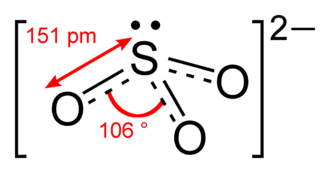
What is the SO3-2 Lewis structure? Where n in this case is 4 since SO3-2 consists of 4 atoms. Therefore, the structure in step 1 is a plausible Lewis structure of SO3-2. Electrons room placed roughly each atom so the the octet dominance is obeyed.
To draw the lewis structure for SO2, we have to find out the valence electrons of sulfur and oxygen first.We express valence electrons as dots in lewis dot structure.
SO₃ stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain. The main use of this component is to make sulfuric acid for industrial purposes.
Lewis structure of SO3. The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.

Draw The Lewis Structure For So3 Determine The Number Of Electron Groups The Electron Geometry And The Molecular Shape Is It Polar Or Nonpolar Study Com

Draw The Lewis Dot Structure For So32 Determine The Electron Geometry And Molecular Shape Of This Molecule Study Com
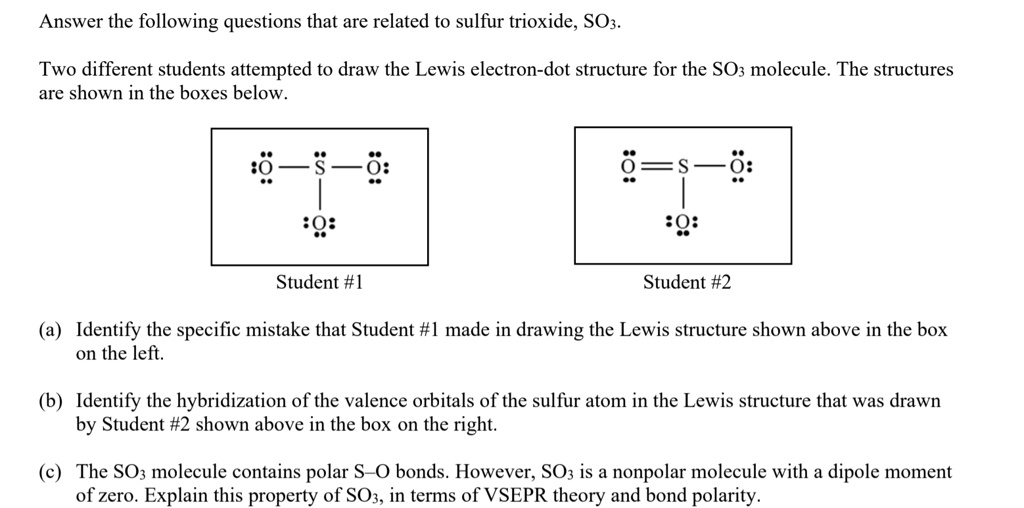
Solved Answer The Following Questions That Are Related To Sulfur Trioxide So3 Two Different Students Attempted To Draw The Lewis Electron Dot Structure For The So3 Molecule The Structures Are Shown In The Boxes









.jpg)






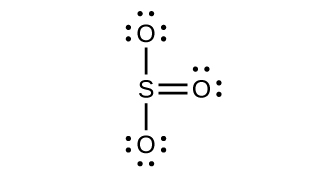








0 Response to "44 lewis diagram for so3"
Post a Comment