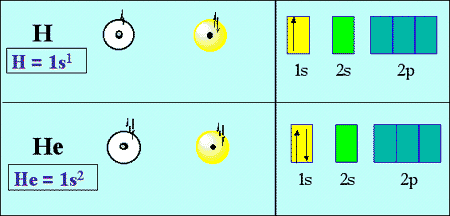
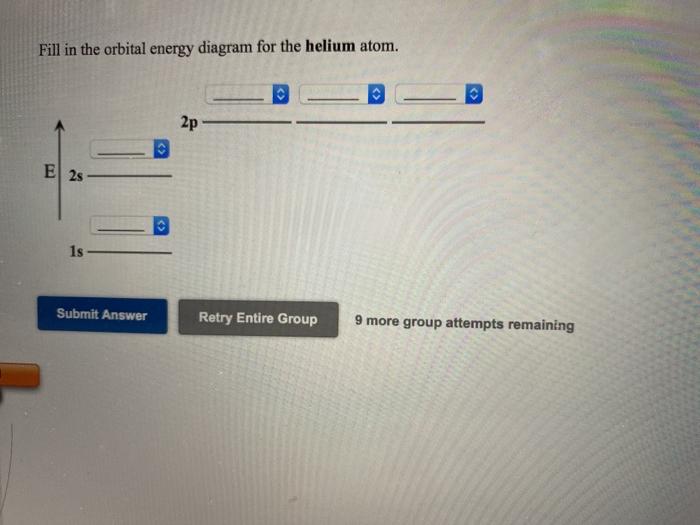
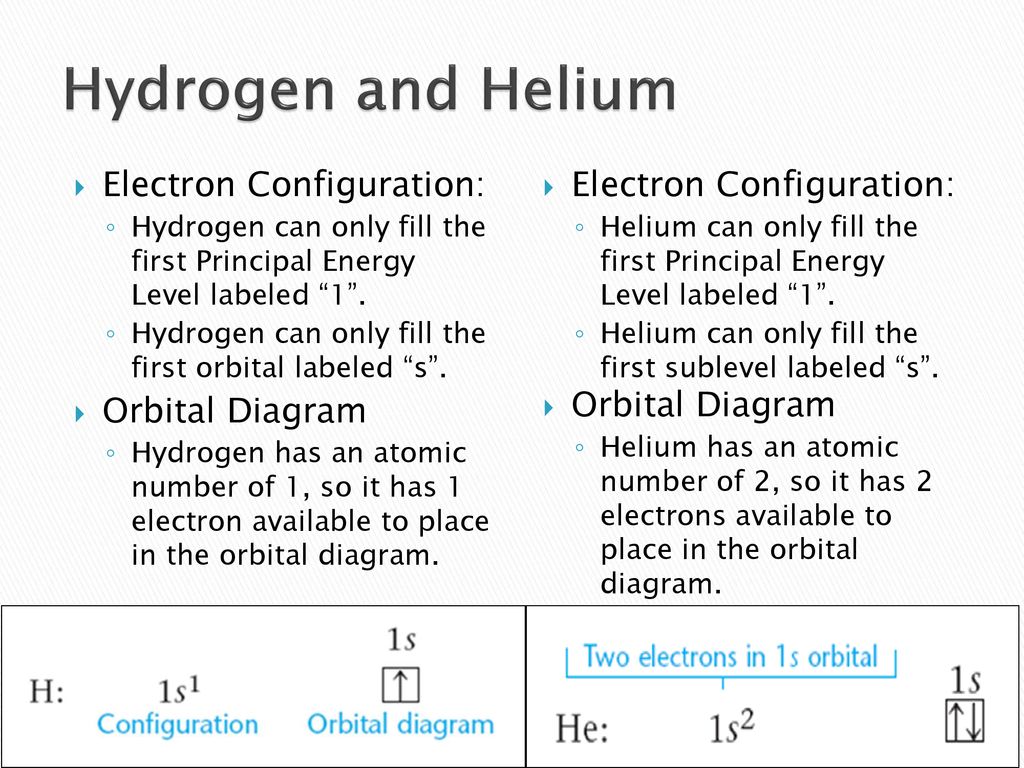
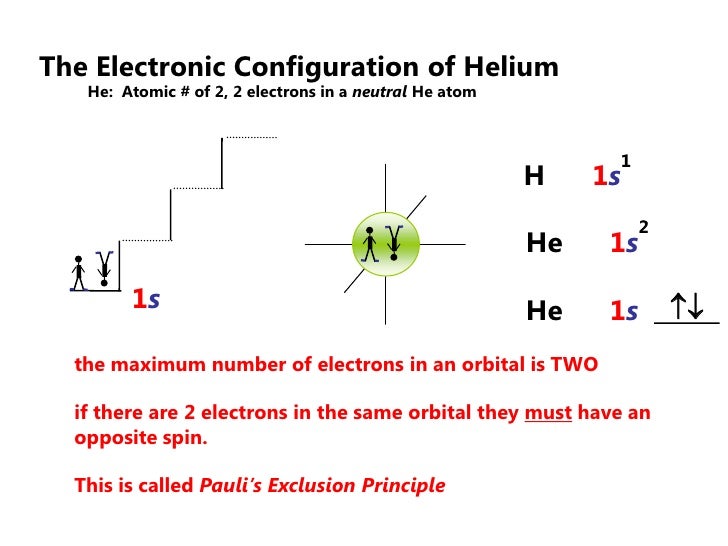
44 use the orbital-filling diagram to show the electron configuration of helium, he.
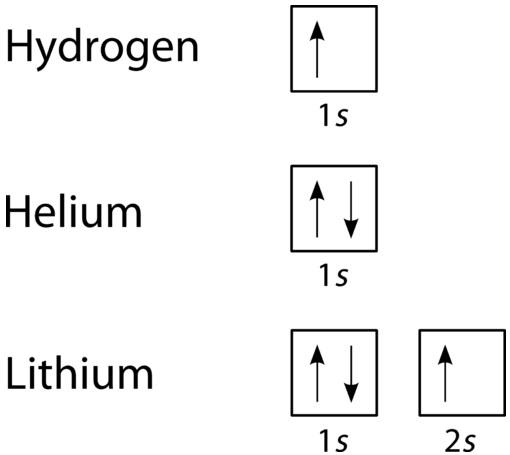
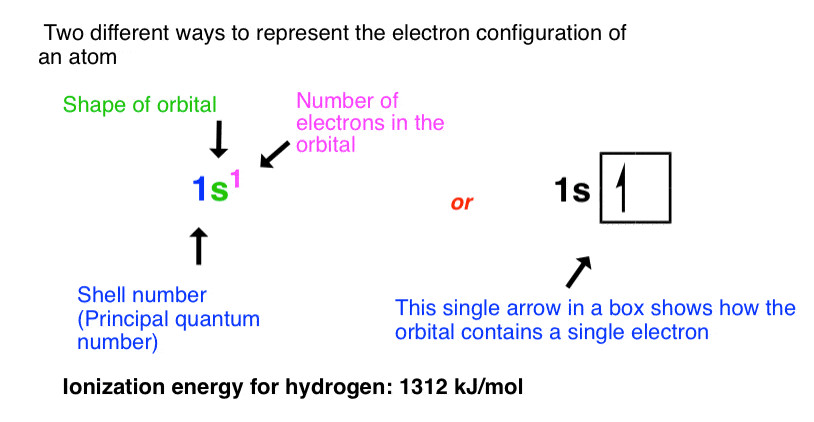
Sample Problem: Orbital Filling Diagrams and Electron Configurations. Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons. Looking at a periodic table you will see that the first period contains only the elements hydrogen and helium. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium And an orbital is a description of that, where is it more or less likely to be found. And this diagram shows us the types of orbitals which can be found...
Helium(He) electron configuration through orbit. Scientist Niels Bohr provided a model of the atom in 1913. The electron configuration of helium shows that the orbit at the end of helium is filled with electrons. Helium does not want to exchange or share any electrons because the last orbit of...

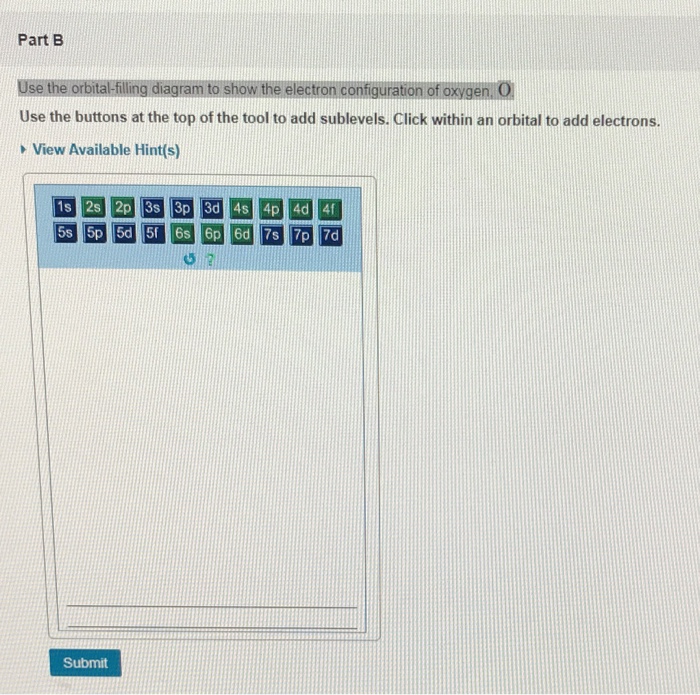
Use the orbital-filling diagram to show the electron configuration of helium, he.
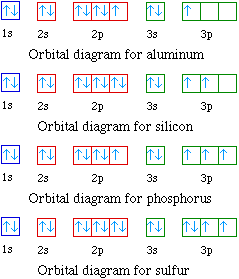
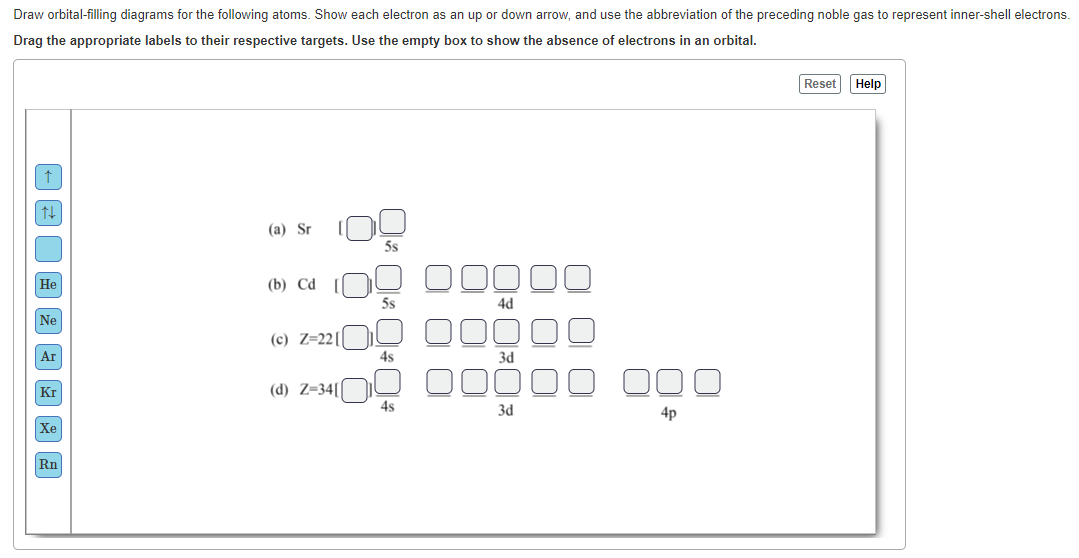
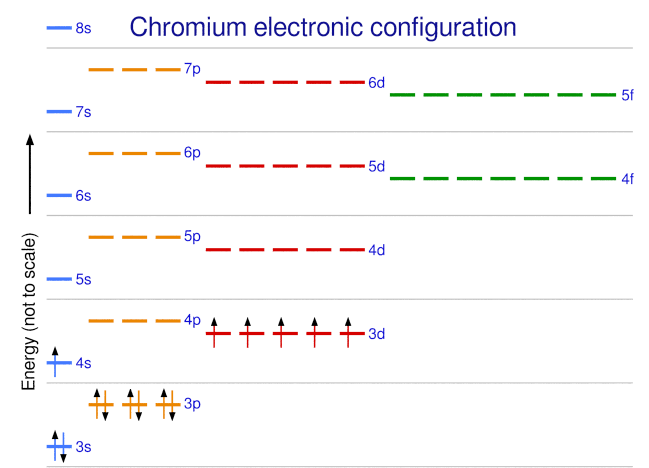
Problem 30 Easy Difficulty. Use the following orbital-filling diagram to show the electron configuration for As Show the orbital filling diagram for br bromine. Stack the subshells in order of energy with the lowest energy subshell at the bottom and the Chapter 8. Chem Ch 2 Flashcards Quizlet. The Electron Configurations Of Atoms. Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And. Electron configurations are the summary of where the electrons are around a nucleus. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them.
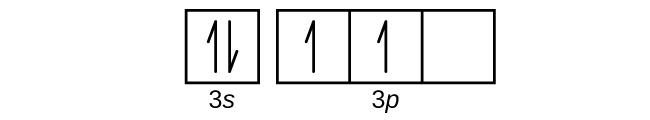
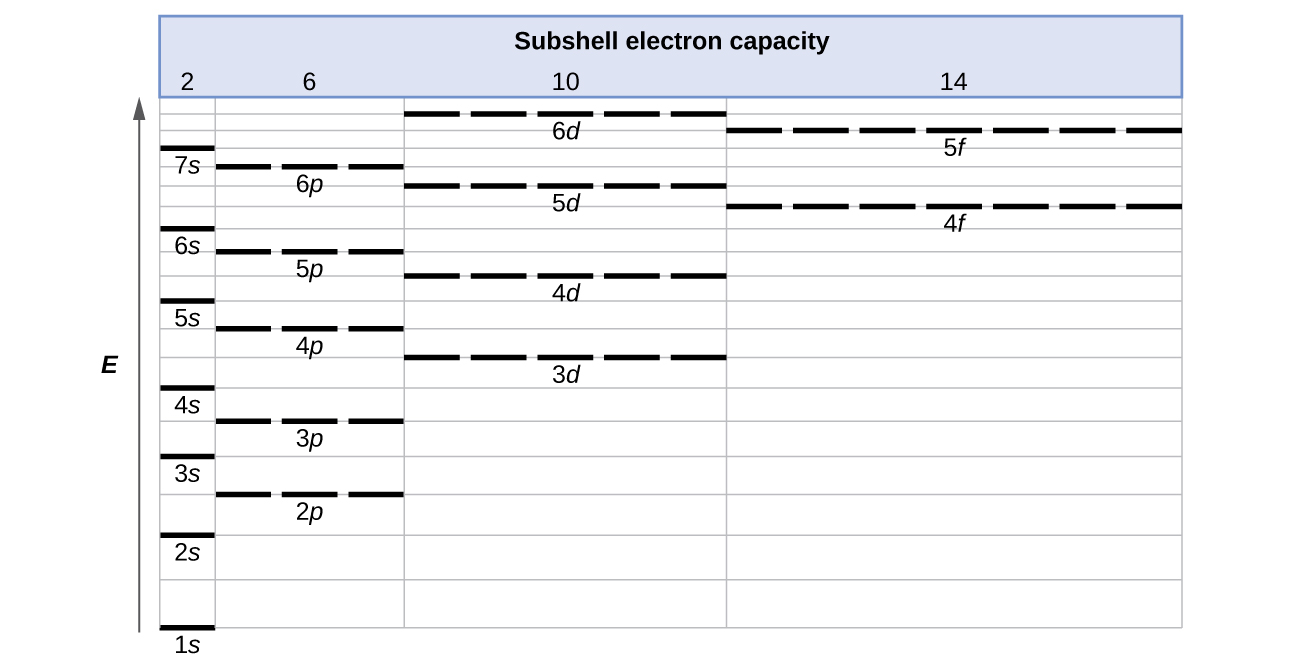
Use the orbital-filling diagram to show the electron configuration of helium, he.. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially Orbital diagrams use the same basic format, but instead of numbers for the electrons, they use ↑ You can use this with any elements apart from hydrogen and helium. Orbital Diagrams. Orbital Filling Electron Configurations. Where do these electrons go?. Orbital Diagrams • Shows the distribution of electrons within orbitals • Circles/Lines/Boxes are used to Orbital Diagram for Helium 1s • Pauli Exclusion Principle • Each orbital can only contain two electrons... An electron configuration shows the distribution of electrons of an atom or a molecule. There is a specific notation that can quickly show you where the electrons are likely to be located, so knowing this notation is an essential part of knowing electron configurations. Chemists use electron configurations to describe the arrangement of electrons around the If you want to write the electronic configuration of an element having atomic number 38 the simle thing declares that the electrons in the orbital are filled up first by the +1/2 spin. Once all the orbitals are...
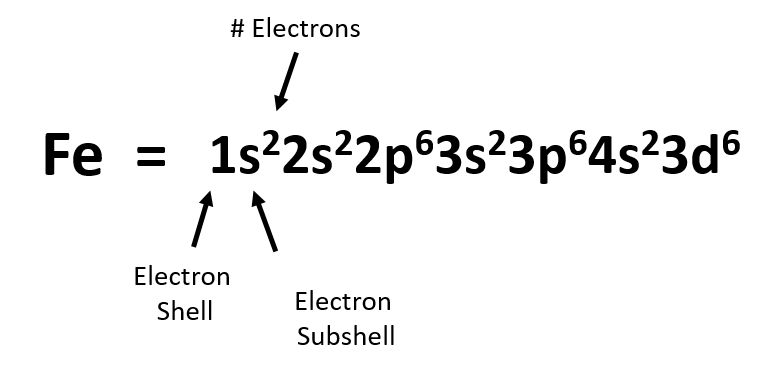
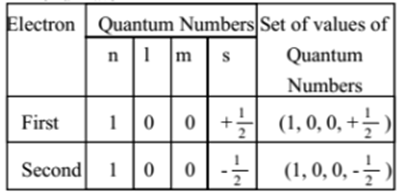
The electron configuration of helium in spdf notation and orbital box notation is therefore Each electron in helium has a unique set of four quantum numbers, as required by the 1 week ago Sep 13, 2020 · Part A Use the orbital-filing diagram to show the electron configuration of helium, He. The electron configuration and orbital diagram of helium are Similarly, the abbreviated configuration of lithium can be represented as [He]2s1, where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium. How to Write Electron Configuration for Helium He Helium He has atomic number 2 and mass number 4. The two electrons are in the same orbit but they orbit in opposite directions they have an opposite spin. Solved Use The Orbital Filling Diagram To Show The Electr Chegg Com. Orbital Diagrams Chemistry Tutorial. Key Concepts. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. Using this information, and the Aufbau Principle, we can write an electron configuration using subshells or sublevels, filling each sublevel...
Electron Configuration. Electrons play a crucial role in chemical reactions and how compounds This is because hydrogen has one electron and helium has two electrons, so we place them in However, we need to show the five different sub-orbitals of the d orbital in order to fully explain... Hello hello I'm has 2 electrons in total and all are in its outer shell. Hope this helps. Which of the following do scientists use to form a hypothesis?  A. A question and observations  B. A conclusion and data  C. A conclusion and a the … ory  D. A question and opinions. Using electron configurations to show how the Periodic Table arises, i.e. an element's position in the Periodic What are the electron configuration (electron arrangement) of 1 Hydrogen, H 2 Helium, He 3 The order of filling the electron levels is listed below and also indicated on the diagram below. Who are the experts?Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.

The Electron Configuration For Helium He Is Shown Below 1s2 Which Diagram Shows The Correct Brainly Com
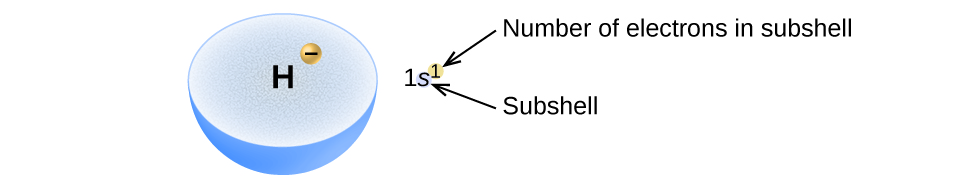
Electron Configurations Worksheet. Examples. Hydrogen (H). Helium (He). Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - can be written using the period table or an electron configuration chart. How to Write the • Helium only has 2 electrons and therefore it has a configuration of 1s 2 . Because the 1s orbital is full with 2...
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram...
The symbols supplied for composing the electron configuration begin with the covering number (n) complied with by the form of orbital and finally the superscript shows how Another way to represent the stimulate of to fill for one atom is by using an orbital diagram often referred to as "the tiny boxes"
Orbital Diagrams Chemistry Tutorial. How. Details: An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an How. Details: Sample Problem: Orbital Filling Diagrams and Electron Configurations. Draw the orbital filling diagram for carbon and write its...
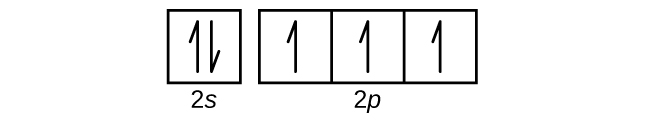
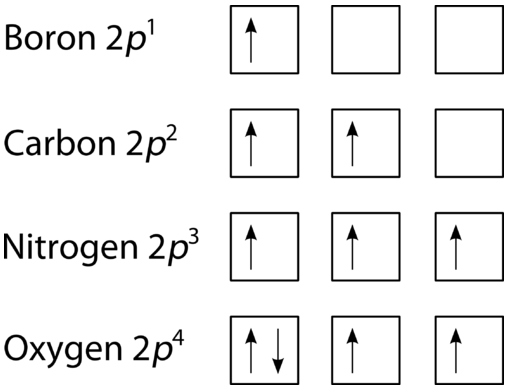
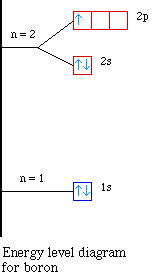
The two electrons present in helium complete the filling of the first shell. This arrangement represents a very stable configuration, as is evidenced by the chemical inertness of helium. Draw the orbital diagram for the electron configuration of oxygen, atomic number 8. How many unpaired electrons...
An orbital diagram helps to determine the electron configuration of an element. Electrons fill orbitals starting at the lowest available energy state before filling higher states. Aufbau procedure: Determine number of electrons for the atom of interest.
We use the orbital energy diagram of Figure 2.1.1, recognizing that each orbital can hold two electrons, one with spin up ↑, corresponding By Hund's rule, the electron configuration of carbon, which is 1s2 2s2 2p2, is understood to correspond to the orbital diagram shown in c. Experimentally...
Use the orbital-filling diagram to show the electron configuration of helium, He. [He]2s2 2p5 Express your answer as a chemical symbol. Enter an abbreviated electron configuration for beryllium: Express your answer in complete form, in order of increasing energy.
Here is an example of orbital configuration for Hydrogen, Helium and Carbon. This can be seen on the orbital filling diagram, but not on the electron configuration which provides less information. The table below shows the electron configuration for the first 20 elements on the periodic table.
Student has agreed that all tutoring, explanations, and answers provided by the tutor will be used to help in the learning process and in accordance with Studypool's honor code & terms of here is the diagram. Please let me know if you need any clarification. I'm always happy to answer your questions.
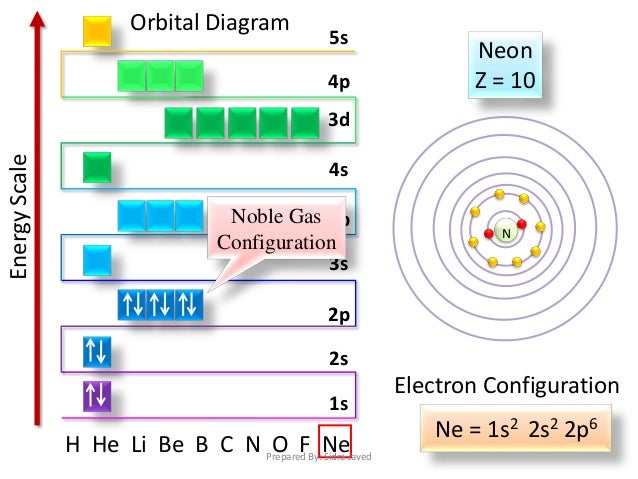
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6, meaning that the 1s...
Therefore, the orbital configuration refers to a layered arrangement of shells, where a specific number of electrons orbit A typical 2n2 notation is used to show the electron configuration. It involves the energy level, orbital number This orbital diagram determines an atom's electron configuration.
Electron configurations are the summary of where the electrons are around a nucleus. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them.
Show the orbital filling diagram for br bromine. Stack the subshells in order of energy with the lowest energy subshell at the bottom and the Chapter 8. Chem Ch 2 Flashcards Quizlet. The Electron Configurations Of Atoms. Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And.
Problem 30 Easy Difficulty. Use the following orbital-filling diagram to show the electron configuration for As



















0 Response to "44 use the orbital-filling diagram to show the electron configuration of helium, he."
Post a Comment