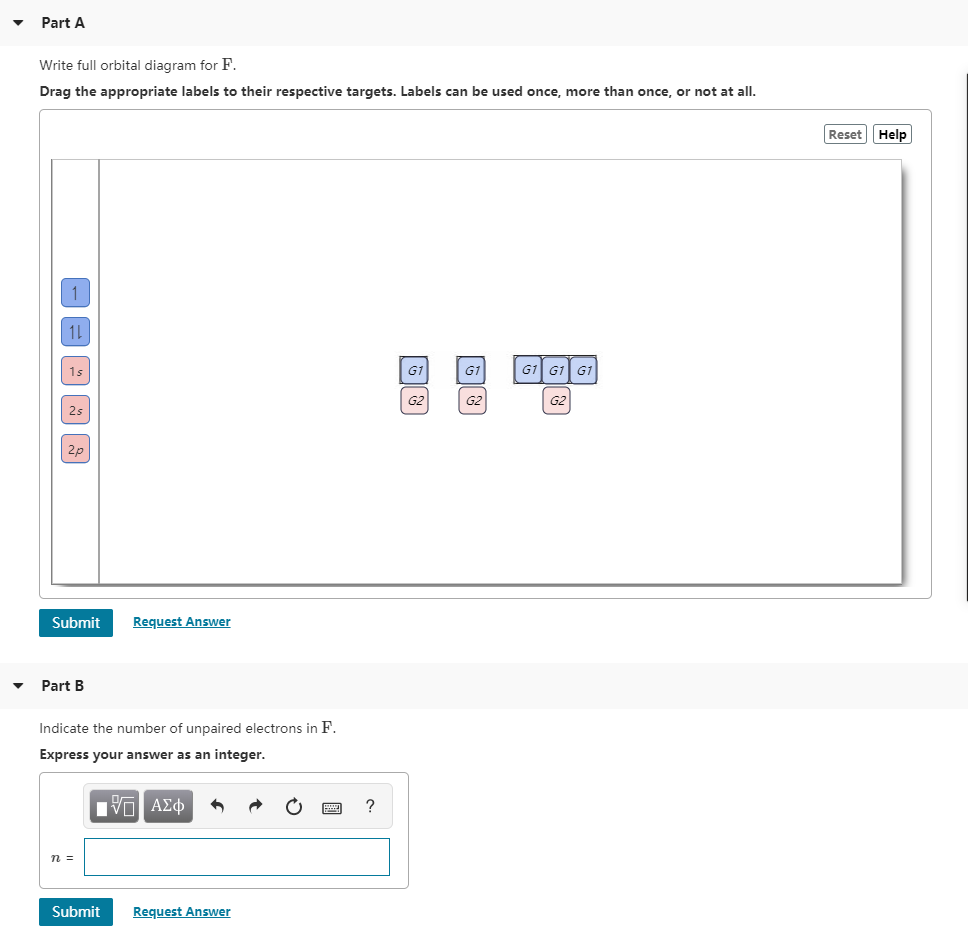
44 write full orbital diagram for f
How to Write an Electron Configuration. The symbols used for writing the electron configuration start with the shell number (n) followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital. For example: Looking at the periodic table, you can see that Oxygen has 8 electrons. An orbital diagram is a different way to show the electron configuration of an atom. It symbolizes the electron as an arrow in a box that represents the orbital. The orbital for a hydrogen atom: (is a box with 1s under it and an H next to it. It has one arrow in it facing up)
Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital. When we write the configuration we ...

Write full orbital diagram for f
Boron electron configuration is 1s 2 2s 2 2p 1.The period of boron is 2 and it is a p-block element. This article gives an idea about the electron configuration of boron(B) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.. The fifth element in the periodic table is the boron(B). Write the full electron configuration for each element. (a) Si (b) 0 (c) K (d) Ne 43. Write the full orbital diagram for each element. (a) N (b)F (c) Mg (d) Al 45. Use the periodic table to write an electron configuration for each element. Represent core electrons with the symbol of the previous noble gas in brackets. Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitrogen 1s 2s 2p 3s 2. Chlorine 1s 2s 2p 3s 3p 3. Sodium 1s 2s 2p 3s 3p 4. Neon 1s 2s 2p 3s 3p 5. Nickel 1s 2s 2p 3s 3p 4s 3d 6) Vanadium 1s 2s 2p 3s 3p 4s 3d 7) Copper 1s 2s 2p 3s 3p 4s 3d
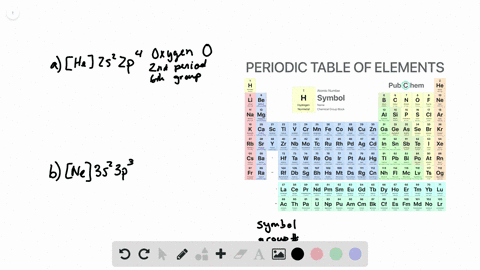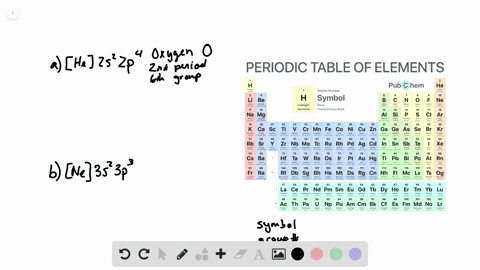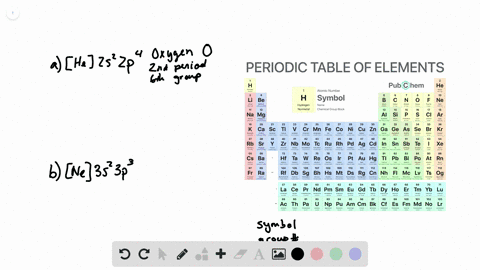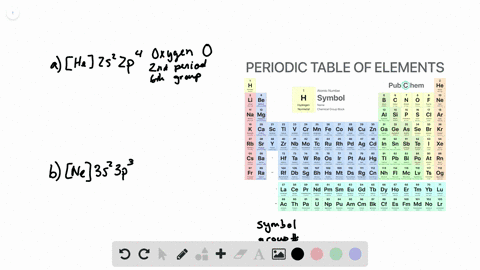
Write full orbital diagram for f. Show the orbital-filling diagram for (bromine).Status: Resolved. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top%(15). 1. Describe the two differences between a 2p x orbital and a 3p y orbital. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will be 1s 2 2s 2 2p 5.
Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Where are the Electrons? Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. The 3dx² - y² orbital looks exactly like the first group, except that that the lobes are pointing along the x and y axes, not between them. The 3dz² looks like a p orbital wearing a doughnut around its waist. f ORBITALS. At the fourth and higher levels, there are seven f orbitals in addition to the 4s, 4p, and 4d orbitals. So, F has one unpaired electron. Therefore, the full orbital diagram of F has 9 electrons. The number of unpaired electron present in F is 1, which is present in the the last 2p orbital. Write the orbital diagram for sulfur and determine its number of unpaired electrons. Electron configuration: 1s2 2s2 2p6 3s2 3p4. Orbital diagram: 1s= 1 up 1 down. 2s= 1 up 1 down. 2p= 1 up 1 down 1 up 1 down 1 up 1 down. 3s= 1 up 1 down. 3p= 1 up 1 down 1 up 1 up. ... Write the full orbital diagram for each element. A.) N B.) F C.) Mg D.) Al.
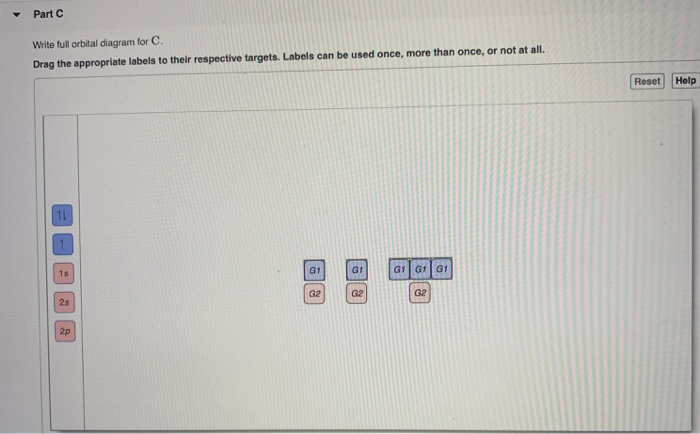
Chemistry. Chemistry questions and answers. Part A Write the full orbital diagram for F. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help 11 1s G1 G1 G1 G1 G1 G2 G2 G2 2s 2p Write the full orbital diagram for C. Drag the appropriate labels to their respective targets. 4. Each sublevel has increasing odd numbers of orbitals available. s = 1, p = 3, d = 5, f = 7. Each orbital can hold only two electrons and they must be of opposite spin. An s-sublevel holds 2 electrons, a p-sublevel holds 6 electrons, a d-sublevel holds 10 electrons, and an f-sublevel holds 14 electrons. The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ... Nov 16, 2021 · A neutral fluorine atom has 9 electrons. Show transcribed image text construct the orbital diagram of the f ion. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbital s in order of increasing energy, starting at the bottom with the. Answer to Write orbital diagram for Co2+. Use the buttons at the top ...
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal –each electron occupies the lowest energy orbital available; German for “build up” •Electrons are notated with an arrow –Up arrow goes first then, down arrow –Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
In writing the electron configuration for Calcium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Calcium go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
For Strontium:a) Write the full electron configuration.b) Write the condensed electron configuration.c) Predict the common ion for Strontium.d) Write the con...
The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
Now that you've mastered the world of electron configurations, it's time to write orbital filling diagrams. This sounds like something that would be tough, but orbital filling diagrams are really just pictures that show you the same thing as electron configurations. Mostly. If you haven't yet learned electron configurations, you really need to go ahead…
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Orbital Diagram For Carbon – Materials Free Full. electron configuration for fluorine f terpconnect electron configuration notation the remaining five electrons will go in the 2p orbital therefore the f electron configuration will be 1s 2 2s 2 2p 5. What Is An Orbital Diagram – Functional Block Diagram Examples Originalstylophone.
Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element.
2. A letter indicates the type of orbital; s, p, d, f. 3. A superscript indicates the number of electrons in the orbital. Example: ls 2 means that there are two electrons in the ‘s’ orbital of the first energy level. The element is helium. How To write an electron configuration: A. Determine the total number of electrons to be represented.
In an orbital (box) diagram a box represents each notation and an orbital diagram. strontium atom (a) in the spdf notation and (b) in the. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of strontium (atomic number: 38), the most.
Solved Getting Started With Pear Gt Mastering Chemistry Ho X Helium Electron Configu X C Complete An Orbital Dia X Chapter 9 Questions Flas X Course Hero
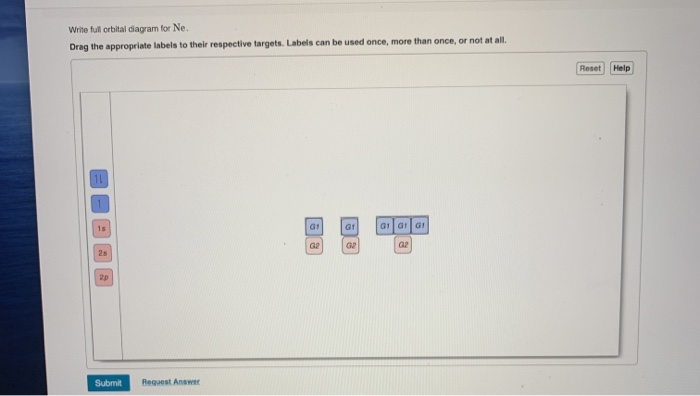
Write the full orbital diagram for Ne. Orbital Diagram: One can easily show the electronic arrangement in different orbitals of an element, using the basic concept of orbital diagram.
Part A Write full orbital diagram for. . Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. ANSWER: Correct Part B Indicate the number of unpaired electrons in it. Express your answer as an integer. ANSWER: Correct Part C Write full orbital diagram for. .
Neon atoms are the 2nd period of the periodic table and an element of the 18-group. The electron configuration of neon ends in a p-orbital. Therefore, it is a p-block element. The melting point of a neon atom is 24.56 K (−248.59 °C, −415.46 °F) and the boiling point is 27.104 K (−246.046 °C, −410.883 °F).
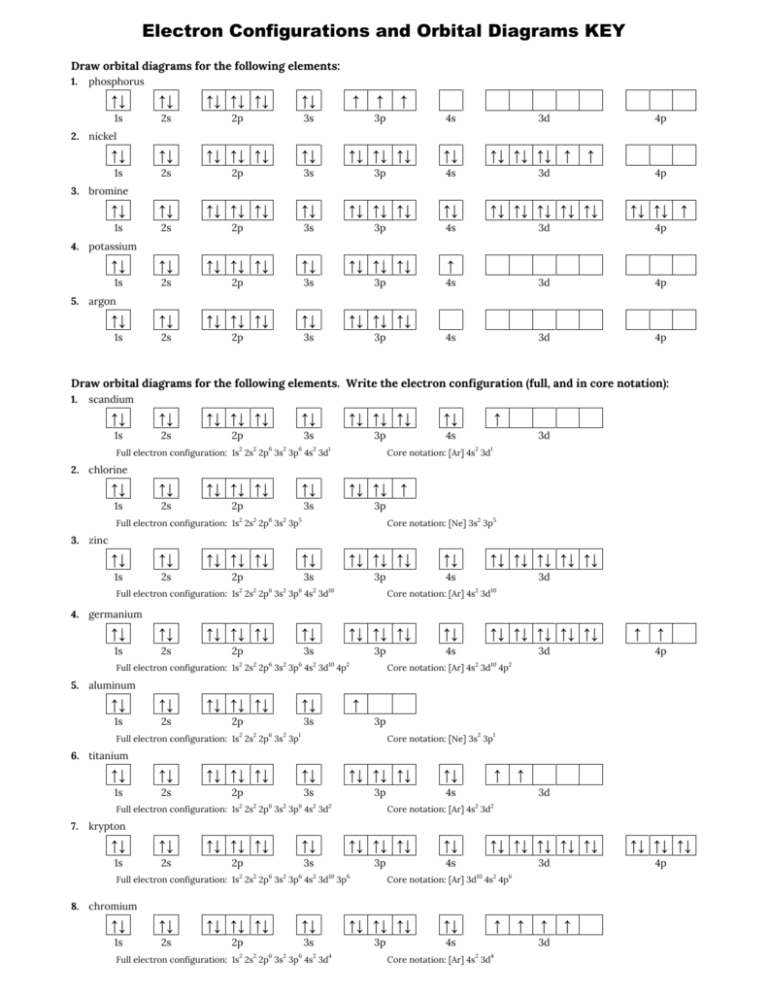
Use this tool to draw the orbital diagram. 3d. 4p. Draw orbital diagrams for the following elements. Write the electron configuration (full, and in core notation). 1. scandium. ↑↓. ↑↓. ↑↓ ↑↓ ↑↓. ↑↓. Contrary to what you may have seen, for Sc and the remaining elements, the 4s is not lower in energy than the 3d. In fact ...
Write the full orbital diagram for element. F. close. Start your trial now! First week only $4.99! arrow_forward. Question. Write the full orbital diagram for element. F. check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here.
Write the full electron configuration, short-hand electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitrogen 1s 2s 2p 3s 2. Chlorine 1s 2s 2p 3s 3p 3. Sodium 1s 2s 2p 3s 3p 4. Neon 1s 2s 2p 3s 3p 5. Nickel 1s 2s 2p 3s 3p 4s 3d 6) Vanadium 1s 2s 2p 3s 3p 4s 3d 7) Copper 1s 2s 2p 3s 3p 4s 3d
Write the full electron configuration for each element. (a) Si (b) 0 (c) K (d) Ne 43. Write the full orbital diagram for each element. (a) N (b)F (c) Mg (d) Al 45. Use the periodic table to write an electron configuration for each element. Represent core electrons with the symbol of the previous noble gas in brackets.
Boron electron configuration is 1s 2 2s 2 2p 1.The period of boron is 2 and it is a p-block element. This article gives an idea about the electron configuration of boron(B) and orbital diagram, period and groups, valency and valence electrons of boron, application of different principles.. The fifth element in the periodic table is the boron(B).

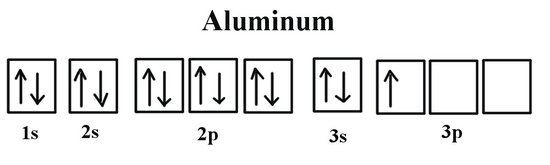
Draw The Partial Valence Level Orbital Diagram And Write The Symbol Group Number And Period Number Of The Element Ar 4s 2 3d 10 4p 3 Image Src Orbital9195593143458043682 Jpg Alt Orbital C Study Com

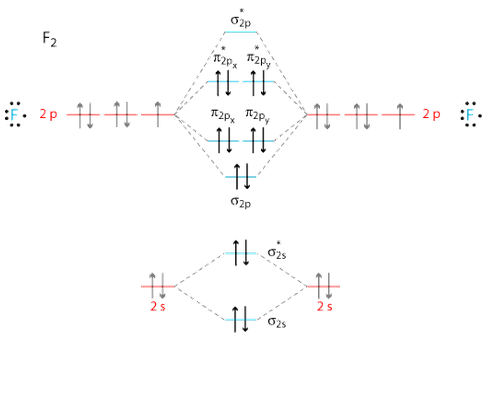
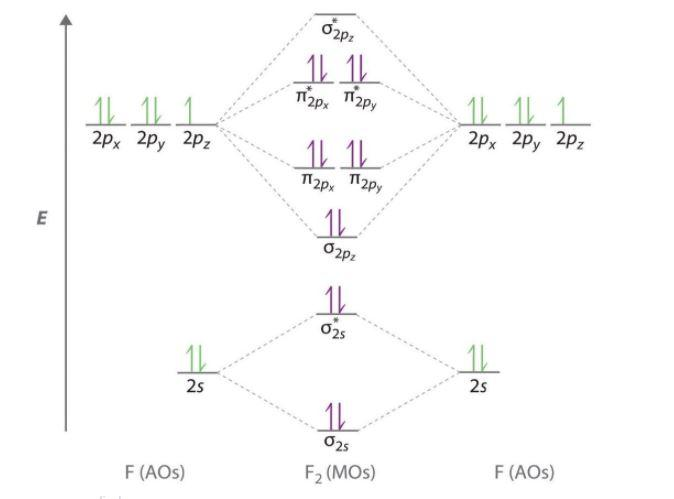
37 Draw Molecular Orbital Diagram For F2 Molecule Also Give Its Electronic Configuration Bond Order And Magnetic Property 138 Solve The Following









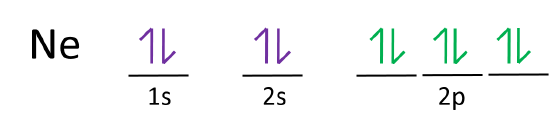










0 Response to "44 write full orbital diagram for f"
Post a Comment