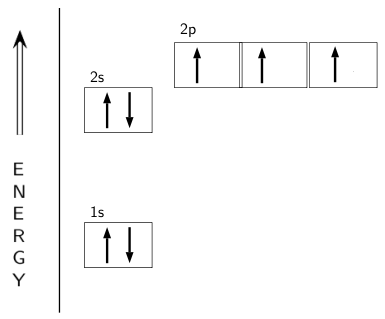
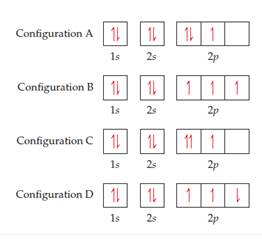
45 the orbital diagram for a ground state nitrogen atom is
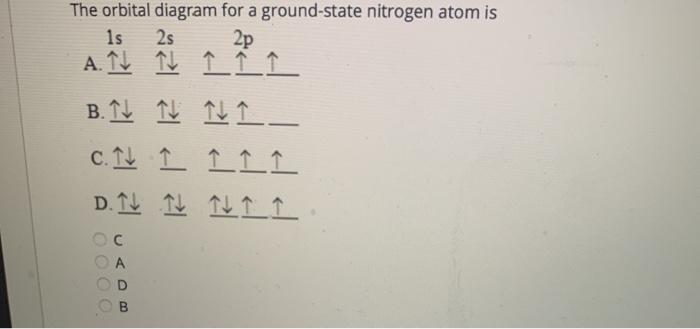
Which orbital notation represents an atom in the ground state with 6 valence electrons? ... Question 56 . SURVEY . 180 seconds . Q. The accompanying diagram represents a portion of a 100-milliliter graduated cylinder. ... Which orbital notation correctly represents the outermost principal energy level of a nitrogen atom in the ground state ... The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소 ; Question: The orbital diagram for a ground-state nitrogen atom is | 1s 25 A.TV C. | | 치서 cm 0 e o000 치어 리 리 리 기 기tmuo | 소
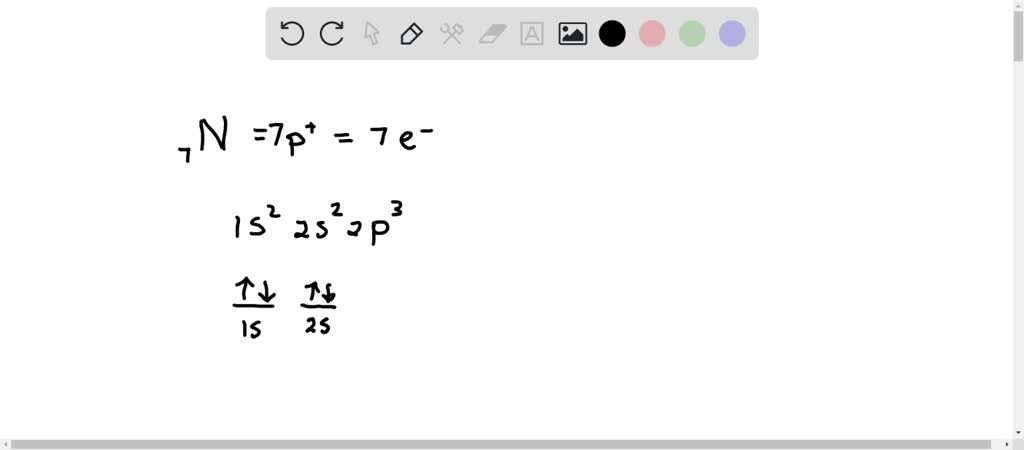
Well, nitrogen's atomic number is 7, so it has 5 valence electrons (two in the 2s orbital and three in the 2p orbitals) and 2 core electrons (two in the 1s orbital). For simple atoms, filling electrons goes in accordance with: The Aufbau principle (lowest to highest energy ordering) Hund's rule (one electron per orbital first, then pair them up after all orbitals for a single energy level are ...

The orbital diagram for a ground state nitrogen atom is
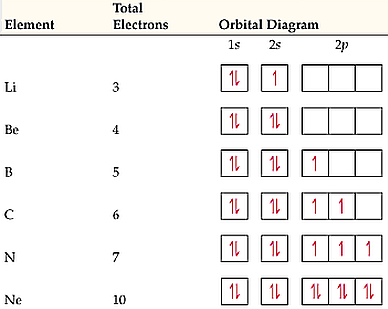
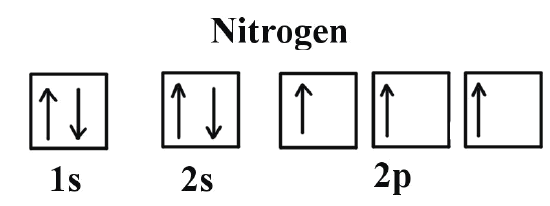
Figure 6.29 tells us that the next lowest power orbital is 2 s, therefore the orbital diagram for lithium is. with 3 unpaired electrons. The electron configuration of nitrogen is therefore 1 s 22 s 22 p 3. At oxygen, v Z = 8 and also eight electrons, we have actually no choice. Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily. The orbital diagram for a ground-state nitrogen atom is. 1s 2s 2p ↑↓ ↑↓ ↑ ↑ ↑. Image: The orbital diagram for a ground-state nitrogen atom is.
The orbital diagram for a ground state nitrogen atom is. here, it states choose the orbital diagram that represents the ground state of nitrogen. Now, if you look at your periodic table, realize that nitrogen is ...Sep 17, 2020 Jan 21, 2021 — 1s22s22p3 is the ground-state electron configuration for N. You can easily understand the image given below. A. Write a ground state electron configuration for each neutral atom. Ground state ... the orbital diagrams, for the following elements. 1. Nitrogen IS 35 2. Chlorine Is 25 3. Sodium Is 2s 35 I 4. Neon The orbital diagram for a ground state nitrogen atom is. C has two unpaired electrons in its ground state. A possible set of quantum numbers for the last electron added to complete an atom of germanium in its ground state is a. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital.
The electron configuration of a ground-state Co atom is ... 1s2,2s2,2p6,3s2,3d9 [Ar]4s2,3d7 [Ne]3s2,3d7 [Ar]4s1,3d5 ... The orbital diagram for a ground-state nitrogen atom is 1s 2s 2p A ↿⇂ ↿⇂ ↿ . ↿ . ↿ B ↿⇂ ↿ . ↿⇂ ↿ . C ↿⇂ ↿⇂ ↿ . ↿ . ↿ Page 2 A)Li B)Si C)Al D)Cl 13.The diagram below represents the orbital notation of an atom's valence shell in the ground state. The diagram could represent the valence shell of The ground state electron configuration of p is ne3s23p3. Electrons in an orbital with l 3 are in a a. Answer to the orbital diagram for a ground state nitrogen atom is. Example k 1s 2 2s 2 2p 6 4s 1 or k. C has two unpaired electrons in its ground state. This subshell is full. Create the atomic orbital diagram for nitrogen. Master chem 1311 ... Writing Orbital Diagrams. We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. We ...
Transcribed image text: Question 21 The orbital diagram for a ground-state nitrogen atom is 1s 2s 2p A. It It Î Î Î B.T ft TuI_ c. ît Î Î Î D. ît îţ îi Î Î ... The orbital diagram for a ground state nitrogen atom. Write the electron configuration for the following elements. The ground state electron configuration of p is ne3s23p3. The remaining three electrons will go in the 2p orbital. You may use the full electron shell notation or the inner shell noble gas method. The orbital diagram for a ground-state nitrogen atom is (2) arrow 1s (2) arrow 2s (3) single arrow 2p. The orbital diagram for a ground state oxygen atom is 2 double arrow-1s; 2 double arrow-2s; 2 double arrow + 2 single arrow -2p. Which ground state atom has an electron configuration described by the following orbital diagram? [Ar] 4s2 3d10 ... orbital diagram for sodium confirms that the 3s sublevel is lower in energy than the 3p sublevel. The s sublevel is located lower on the page than the p sublevel. 10. The lowest potential energy arrangement of electrons in an atom is called the ground state. Ground state electron configurations can be predicted by a strict set of rules known as the
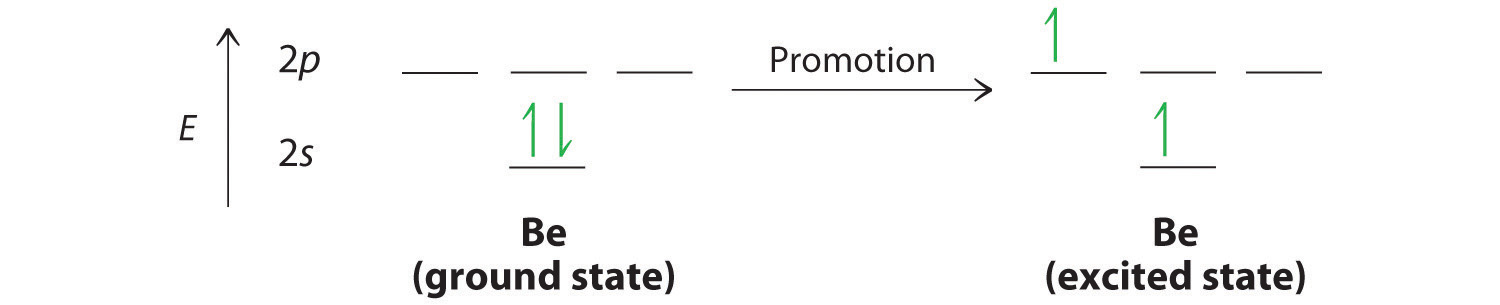
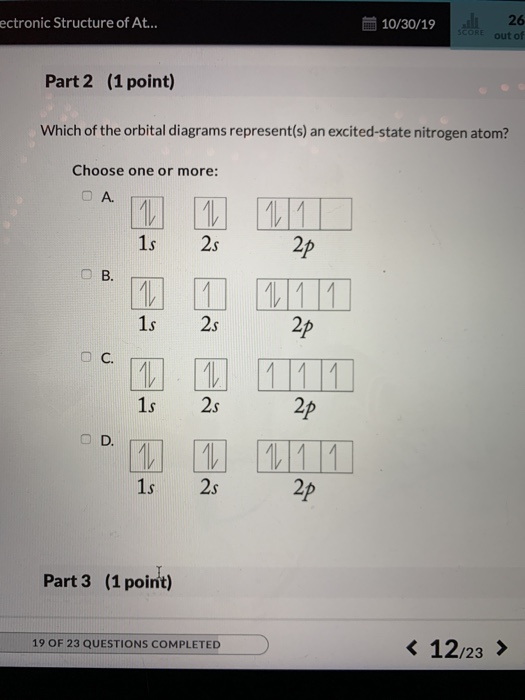
1 answerElectronic configuration of nitrogen in ground state is 1s2 2s2 2p3 or 1s2 2s2 2px1 2py1 2pz1. Hence, in excited state one of the 2s electron will jump to 2p ...
33. "No two electrons in an atom can have the same four quantum numbers" is a statement of A. the Pauli exclusion principle. B. Bohr's equation. C. Hund's rule. D. de Broglie's relation. E. Dalton's atomic theory. 34. The orbital diagram for a ground-state nitrogen atom is A. Row 1. B. Row 2.
Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom. Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1.
The orbital diagram for a ground state nitrogen atom. School San Francisco State University. Course Title PHYS 240. Uploaded By AdmiralKoala1822. Pages 5. This preview shows page 3 - 4 out of 5 pages. View full document. See Page 1. 1 | P a g e INTON MALPAS MO BASAEN TOY MODULE 2 ANSWERAM TO DETOY FIRST SUMMATIVE TEST.
Figure \(\PageIndex1\): One electron in. From the orbital diagram, we can write the electron configuration in one abbreviated type in i m sorry the populated orbitals are determined by their principal quantum number n and their worth of l (s, p, d, or f), v the number of electrons in the subshell indicated by a superscript.For hydrogen, therefore, the solitary electron is inserted in the 1s ...
The orbital diagram for a ground state nitrogen is a a b b c c d d the electron configuration of an atom shows a the number of isotopes possible. C has two unpaired electrons in its ground state. The following orbital filling diagram represents an excited state rather than the ground state of an atom. It depends on the atom.

Orbital Hybridization A Key Electronic Factor In Control Of Structure And Reactivity Alabugin 2015 Journal Of Physical Organic Chemistry Wiley Online Library
Select an atom from the list (you will probably want to do lower atomic numbers). Leave the number set a zero. Look up the atom on a periodic table and determine the number of electrons present. Draw the orbital filling diagram for the atom. Click on the "Calculate" button and compare your answer with the one provided.
What orbital diagram correctly represents the outermost principal energy level of a nitrogen atom in the ground state? 1S22S23P3 1S22S23P3 What atom is represented in the following orbital diagram ...
Oct 24, 2021 · 4 answersHi there. In this question, we are trying to show the orbital diagram for A nitrogen atom and nitrogen is atomic # seven.
The orbital diagram for a ground-state nitrogen atom is. 1s (up down) 2s (up down) 2p (up, up, up) Electrons in an orbital with l = 3 are in a/an. ... For all atoms of the same element, the 2s orbital is larger than the 1s orbital. true. An FM radio station broadcasts at a frequency of 101.7 MHz. Calculate the wavelength
3 Unpaired electrons. Nitrogen atom has total 7 electrons. Two will fill up the n=1 level, and then there are five electrons in the n=2 level. Nitrogen can bond three times with other electrons to fill up it's shell with 8, (8-5=3). And these are those 3 unpaired electrons which were residing the 2p sub-shell of the Nitrogen atom , before the formation of 3 bonds.
The orbital diagram for a ground-state nitrogen atom is. 13. The orbital diagram for a ground-state oxygen atom is. 14. The orbital diagram for a ground state carbon atom is. 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these ...
Ground or Excited State for Nitrogen. In question 1.69 (b), there is a picture which shows the electron configuration for Nitrogen. There are two arrows for the 1s orbital, 2 arrows in the 2s orbital, and one arrow in each of the three 2p orbitals. The question asks us to determine whether the electron configuration represents the excited state ...
The orbital diagram for a ground state nitrogen is a a b b c c d d the electron configuration. It depends on the atom. Since 1s can only hold two electrons the next 2 electrons for n goes in the 2s orbital. The ground state electron configuration of p is ne3s23p3. Nitrogen is the seventh element with a total of 7 electrons.

The Ground State Valence Shell Electrons Configuration Of Nitrogen Atom Can Be Represnted As Youtube
The orbital diagram for a ground-state nitrogen atom is. Ans: A. Category: Medium Section: 7.8. 41. The orbital diagram for a ground-state oxygen atom is. Ans: D. Category: Medium Section: 7.8. 42. The orbital diagram for a ground state carbon atom is. Ans: D. Category: Medium Section: 7.8. 43. Which ground-state atom has an electron ...
Chemistry questions and answers. The orbital diagram for a ground-state... The orbital diagram for a ground-state oxygen atom is в. 1, т. 11- С.Т 1Тут — D. 1 1 1 1 1 E. Тут, ТІ ТІ ооооо.
The orbital diagram for a ground state oxygen atom is. The orbital diagram for a ground state oxygen atom is. Because oxygen has 8 electrons its configuration is 1s2 2s2 2p4. The ground state electron configuration of p is ne3s23p3. The colors of the visible spectrum are red orange yellow green blue and violet.
The orbital diagram for a ground state nitrogen atom is. Write the ground state electron configuration and orbital notation for each of the following atoms. A possible set of quantum numbers for the last electron added to complete an atom of germanium in its ground state is a. 1b a energy is absorbed b light is emitted c the electron can have a ...
The orbital diagram for a ground-state nitrogen atom is. 1s 2s 2p ↑↓ ↑↓ ↑ ↑ ↑. Image: The orbital diagram for a ground-state nitrogen atom is.
Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
Figure 6.29 tells us that the next lowest power orbital is 2 s, therefore the orbital diagram for lithium is. with 3 unpaired electrons. The electron configuration of nitrogen is therefore 1 s 22 s 22 p 3. At oxygen, v Z = 8 and also eight electrons, we have actually no choice.



















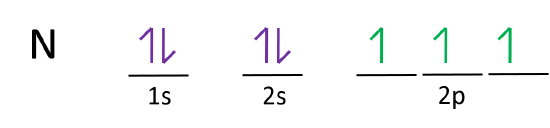

0 Response to "45 the orbital diagram for a ground state nitrogen atom is"
Post a Comment