45 potential energy diagram endothermic
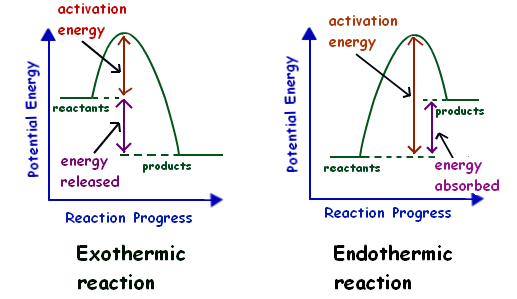
Exothermic and Endothermic Potential Energy Diagrams There are two types of potential energy diagrams. These two types center on the difference between the energies of the reactants and products. Consider the figure below. An endothermic reaction is shown on the left, and an exothermic reaction is shown on the right. ... Compare the slope of the line on a potential energy diagram for an endothermic reaction and an exothermic reaction. exothermic is very similar to the potential energy diagram and endothermic is a flipped picture of the potential energy diagram. FQ: How does activation energy affect the outcome of chemical reactions?
How does the potential-energy diagram for a reaction indicate whether the reaction is endothermic or exothermic? A. An endothermic reaction has reactants that are lower in energy than products because energy is absorbed to form the products.
Potential energy diagram endothermic
Increase in kinetic energy - energy absorbed - endothermic Decrease in kinetic energy - energy released - exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ... For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential energy diagram represents an endothermic reaction? Potential Energy Potential Energy - ЕЛИ Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit.
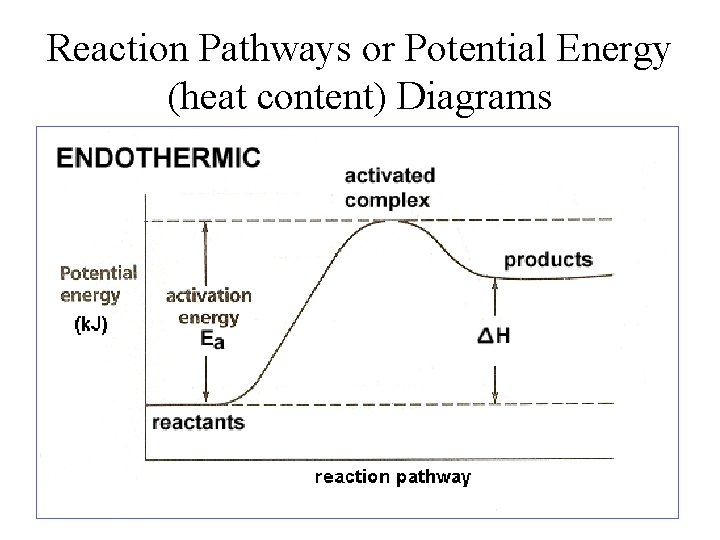
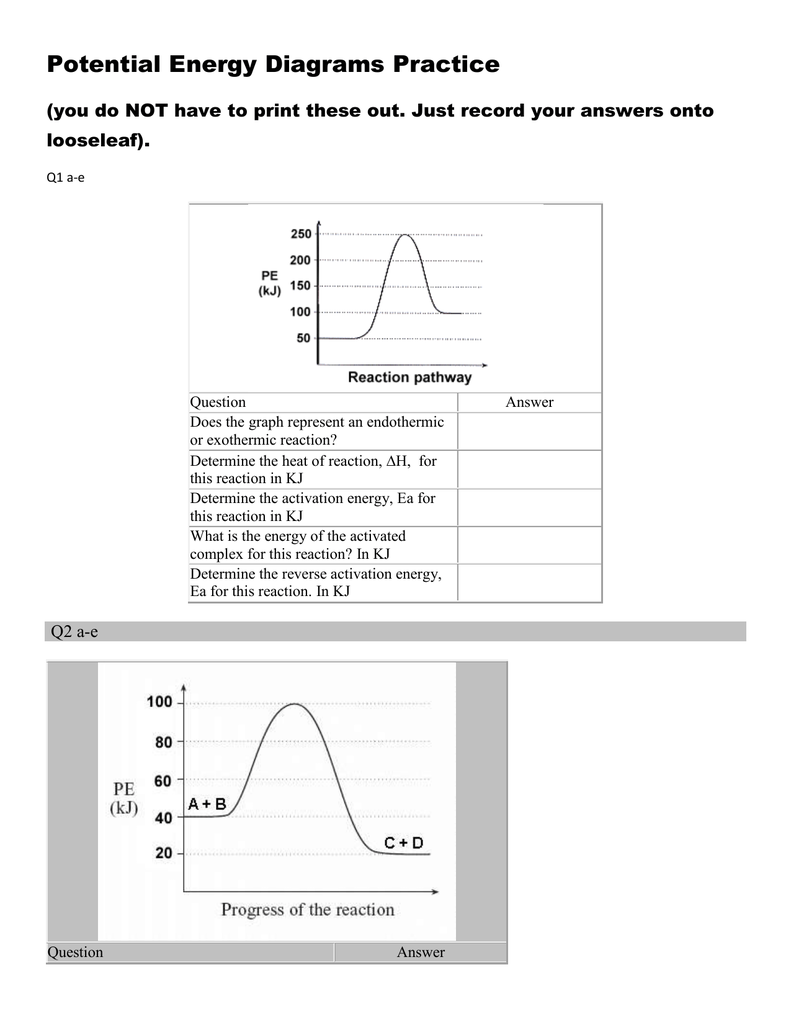
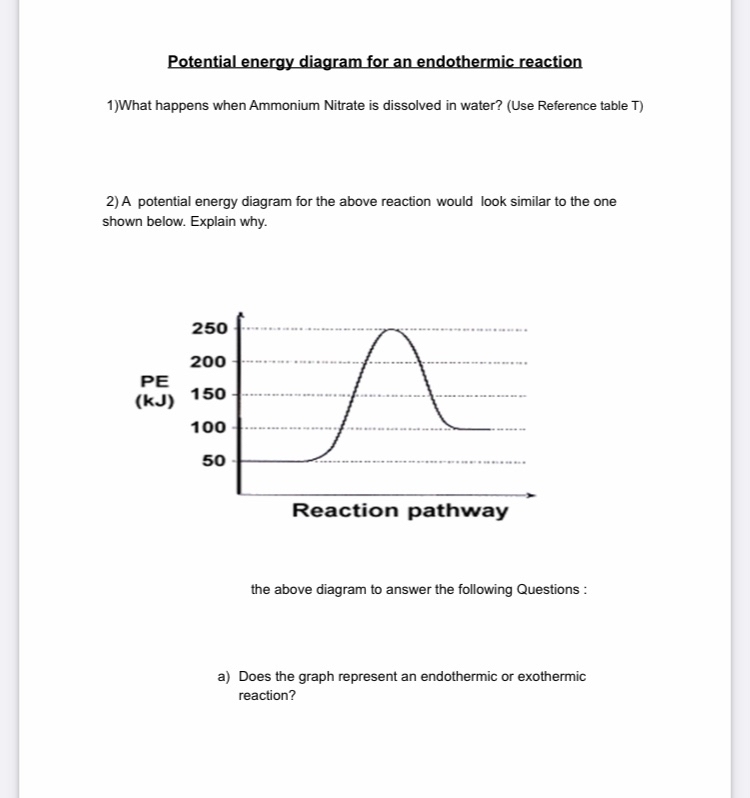
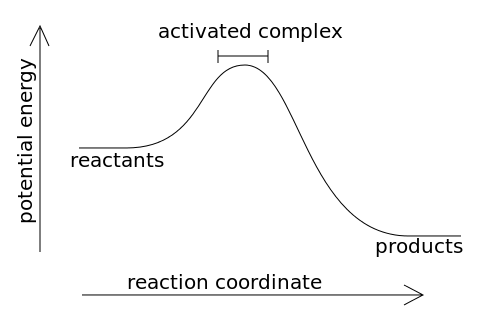
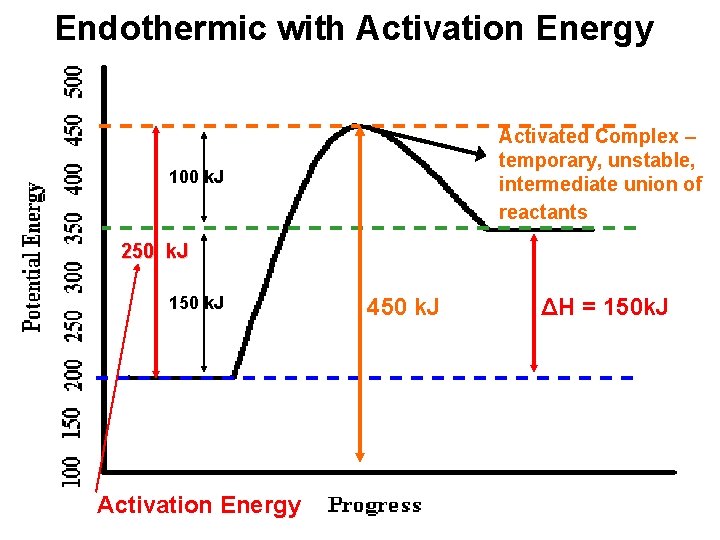
Potential energy diagram endothermic. This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. Figure shows the energy level diagram of an exothermic reaction. Figure shows the energy level diagram of an endothermic reaction. Draw a dashed line on the diagram to indicate a potential energy curve for the reaction if a catalyst is added. 250 200 150 (kJ) 100 50 2) Sketch a potential energy curve below that is represented by the following values of AH and Ea (activation energy). You may make up appropriate values for the y-axis (potential energy). AH = -100 Id and Ea ... Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Energy reactants products exothermic reactions the reactants have more potential energy than the ...
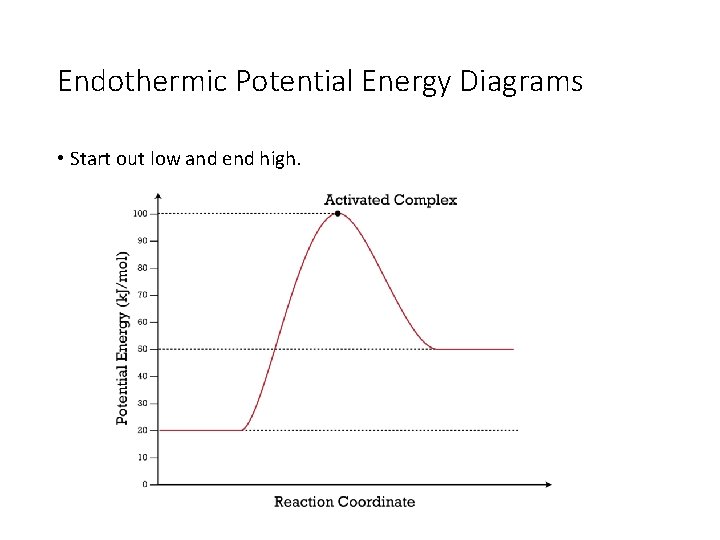
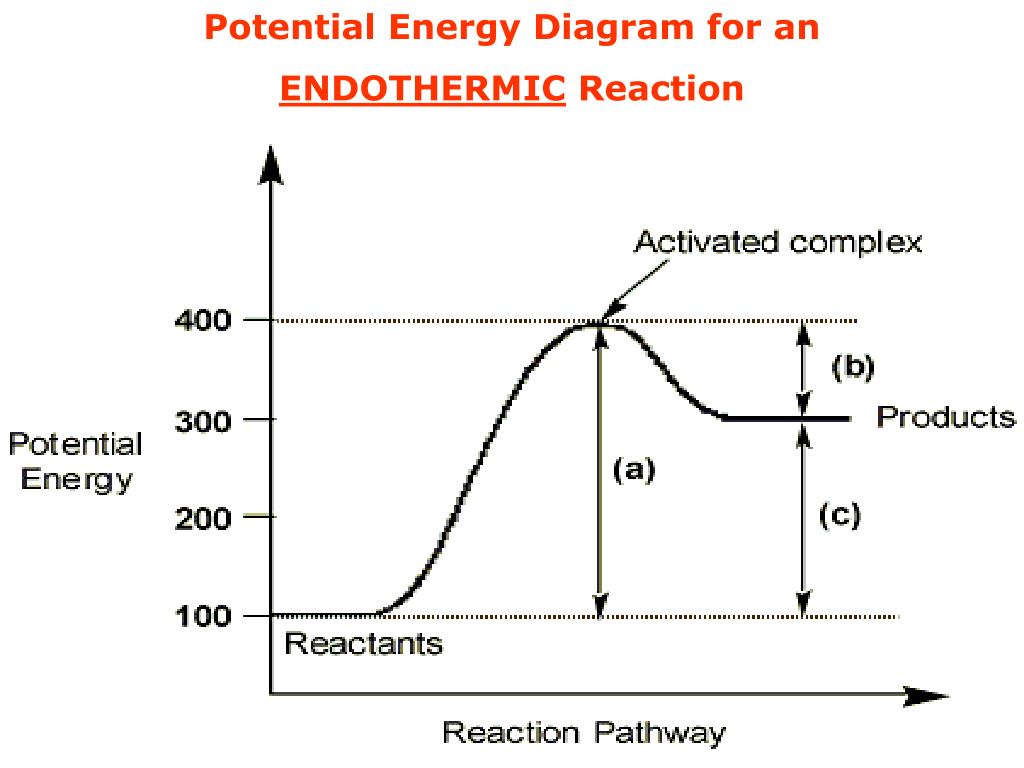
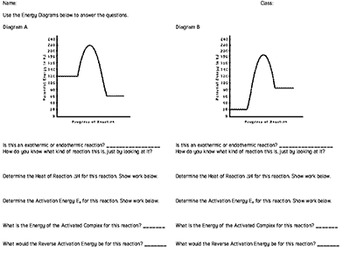
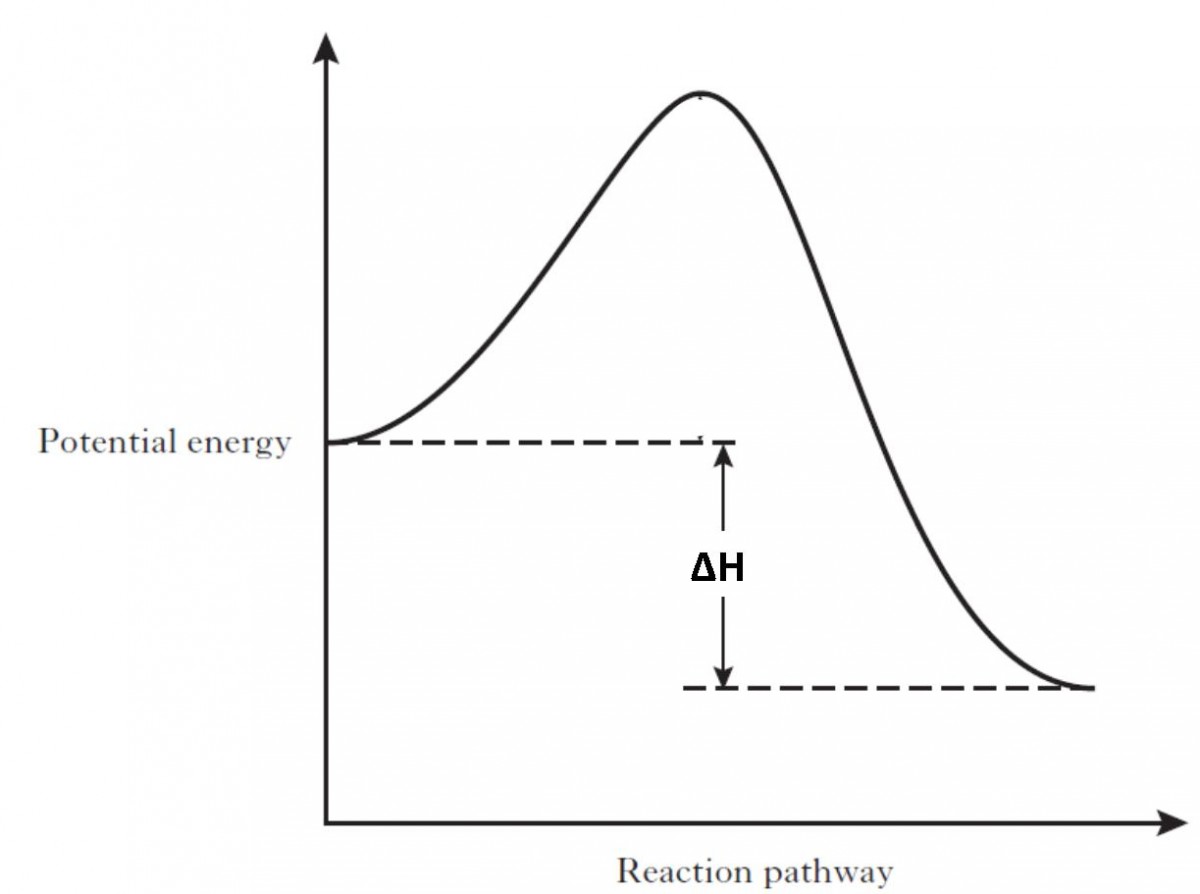
74. B6 Consider the following potential energy diagram: The above potential energy diagram represents an: A. exothermic reaction involving one step. B. exothermic reaction involving two steps. C. endothermic reaction involving one step. D. endothermic reaction involving two steps. 75. B6 Consider the following reaction: The activation energy for the formation of HI is 167 kJ. Students will be given two Potential Energy Diagrams -- one Endothermic, one Exothermic. They will have to read the graph and determine if the graph is showing an endothermic or exothermic reaction, justify why it is so, then calculate the heat of reaction (delta H), activation energy (Ea), reverse Potential energy diagrams. Chemical reactions involve a change in energy, usually a loss or gain of heat energy. The heat stored by a substance is called its enthalpy (H). \ (\Delta H\) is the ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants.
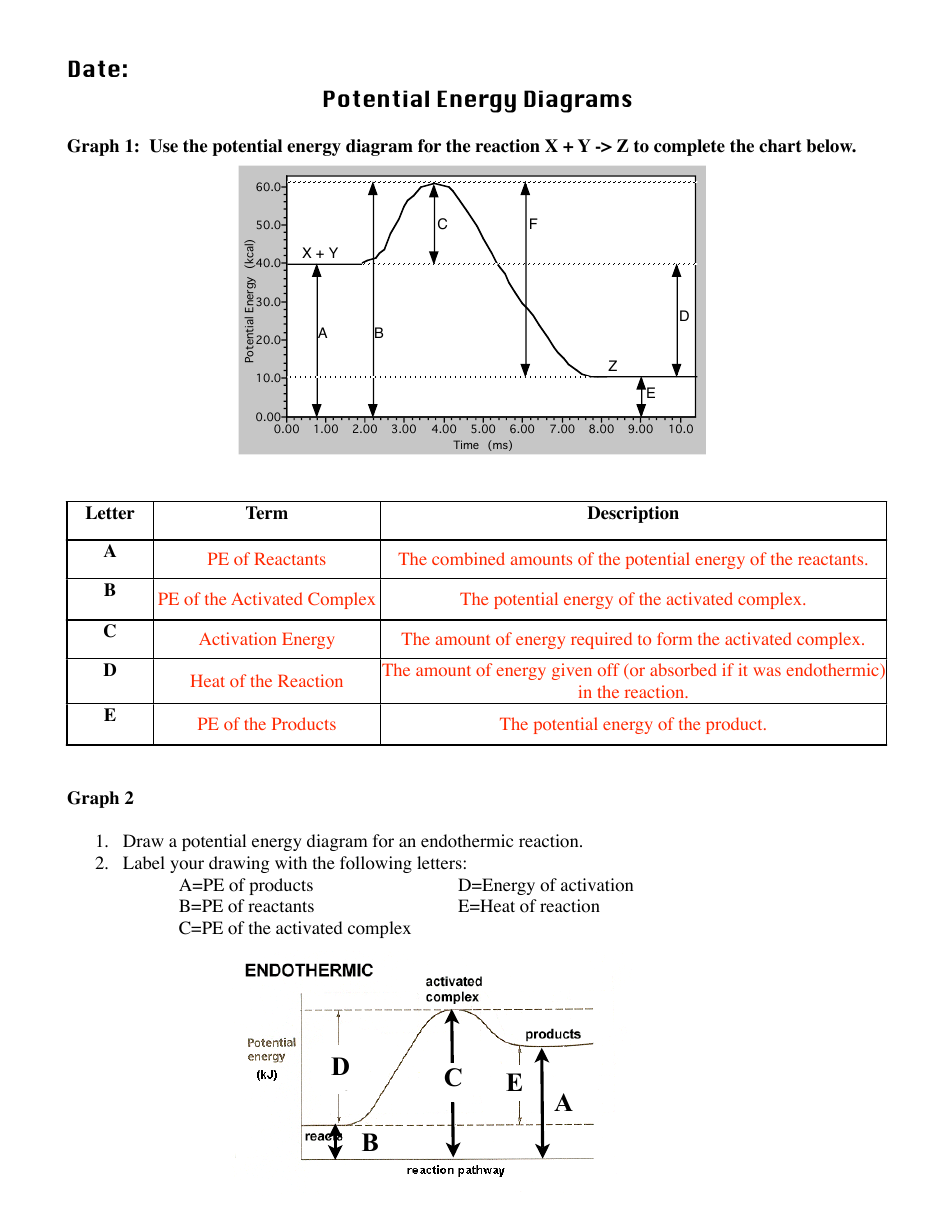
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction ... A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and ΔH is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants and ΔH is ... Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ... 3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement. Reaction I Reaction 2 a) E P of the reactants C R b) E P of the products E Z c) ∆H of the reaction B S d) activation energy of the
State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer--
Solved A Student Made A Sketch Of A Potential Energy Diagram To Represent An Exothermic Reaction Explain Using Complete Sentences Why The Diagra Course Hero
There are other types of energy which may be produced or absorbed by a chemical reaction. Examples include light and sound. In general, reactions involving energy may be classified as endergonic or exergonic, An endothermic reaction is an example of an endergonic reaction. An exothermic reaction is an example of an exergonic reaction.
Thermodynamics Review 27. Given the potential energy diagram: With reference to energy, the reaction A + B → AB can best be described as 1) endothermic, having a +ΔH 2) endothermic, having a -ΔH 3) exothermic, having a +ΔH 4) exothermic, having a -ΔH 28.
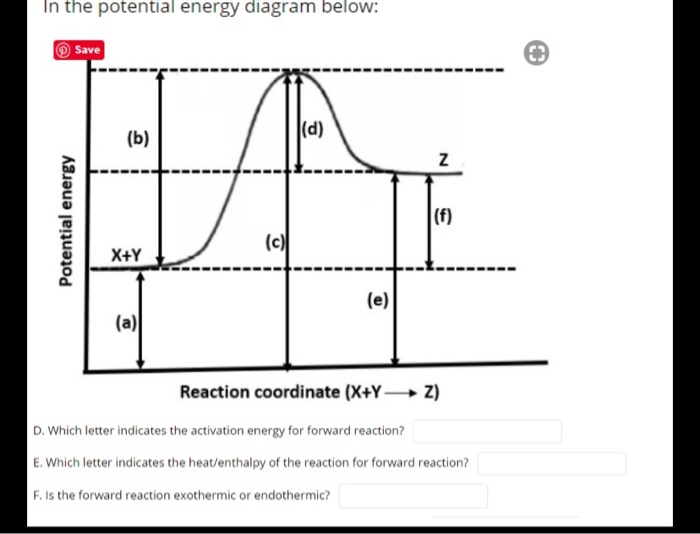
ASSESSMENT - POTENTIAL ENERGY DIAGRAM 1. Is the reaction endothermic or exothermic? 2. What letter represents the potential energy of the reactants? 3. What letter represents the potential energy of the products? 4. What letter represents the change in energy for the reaction? b f d 11. ASSESSMENT - POTENTIAL ENERGY DIAGRAM 5.
Representing endothermic and exothermic processes using energy diagrams. AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. If the initial state has a lower potential ...
52 Sketch the potential energy diagram for an endothermic chemical reaction that shows the activation energy and the potential energy of the reactants and the potential energy of the products. Answer--> 1/04. 16 Which statement best explains the role of a catalyst in a chemical reaction?
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
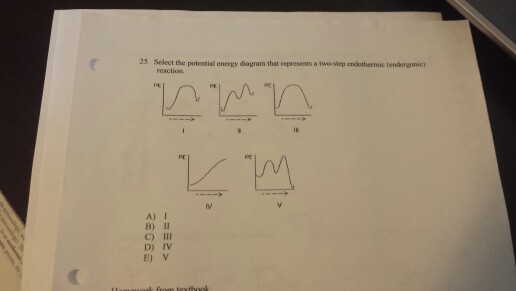
Select the potential energy diagram that represents a two-step endothermic (endergonic) reaction. I II III IV; Question: Select the potential energy diagram that represents a two-step endothermic (endergonic) reaction. I II III IV. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Endothermic reactions: Heat is absorbed. 1) Photosynthesis: Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. 6CO2 + 6 H2O + heat ---> C6H12O6 + 6O2. 2) Cooking an egg: Heat energy is absorbed from the pan to cook the egg.
For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential energy diagram represents an endothermic reaction? Potential Energy Potential Energy - ЕЛИ Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit.
The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
Increase in kinetic energy - energy absorbed - endothermic Decrease in kinetic energy - energy released - exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds.

Based On Their Activation Energies And Energy Changes And Assuming That All Collision Factors Are The Same Which Of The Following Reactions Would Be Fastest In The Reverse Direction A Ea

Potential Energy Diagrams For A Given Reaction The Activation Energy And The Enthalpy Of Reaction Can Be Shown Graphically In A Potential Energy Diagram Ppt Download
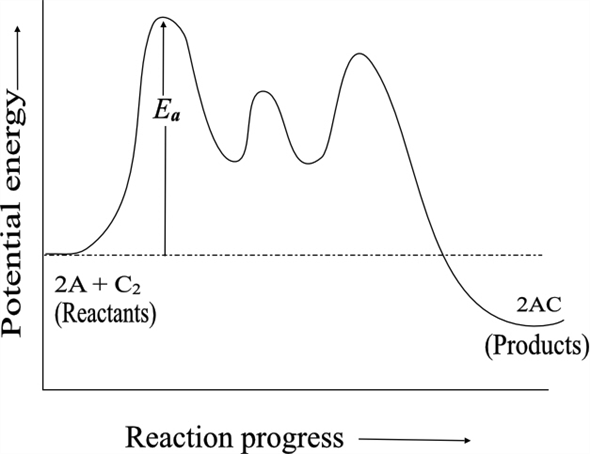
Solved Chapter 13 Problem 32p Solution Chemistry Plus Masteringchemistry With Etext Access Card Package 7th Edition Chegg Com
Solved A Student Made A Sketch Of A Potential Energy Diagram To Represent An Exothermic Reaction Explain Using Complete Sentences Why The Diagra Course Hero


















0 Response to "45 potential energy diagram endothermic"
Post a Comment