40 calculate bond order from mo diagram
In Molecular orbital diagram, we just need to calculate the number of electrons in anti-bonding orbital and bonding orbital, then we can use the formula in order to calculate bond order is: Bond order = (No. of electrons in anti-bonding MO) - (No. of electrons in bonding MO) / 2. Hope this helps! CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape. CS2 is an abbreviated form of Carbon Disulphide. This molecule has two Sulphur atoms and one Carbon atom. To understand the hybridization, molecular geometry and the polarity of this molecule it is essential to under its Lewis structure. ぷっくりお花のキーホルダー ...
For the bond order formula of simple molecules and ions, draw the molecular orbital diagram and determine the number of bonding and antibonding electrons. Then calculate the bond order with the ...

Calculate bond order from mo diagram
We use molecular orbital theory to calculate the bond order between two atoms. It's very interesting to draw the energy level diagram of the atoms. Read more What is the Bond Order in F2? Answer- 1 is the bond order for an f2 molecule. We calculate the bond order with the help of molecular orbital theory or bond order theory. The double bond of butane is composed of a π bond formed by the overlap of p orbitals and a σ bond formed by the overlap of sp2 hybrid orbitals. A 90-degree rotation about the bond eliminates all overlap of the p orbitals that form the π bond and it is broken. In molecular orbital theory, bond order is also defined as half of the difference between the number of bonding and antibonding electrons.Dec 5, 2017 · Uploaded by chemistNATE
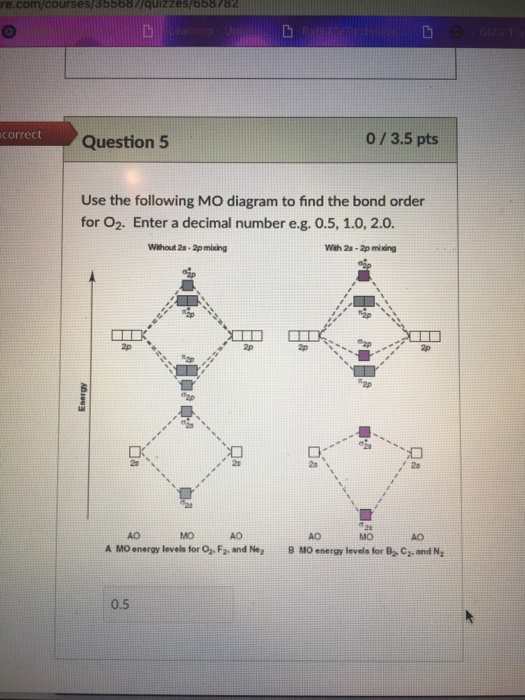
Calculate bond order from mo diagram. Answer (1 of 3): I modified the picture from this post: What's the MOT diagram of O2 +2 ion? and modified it to be O2 2+ (since sadly enough I am about as advanced with artistic programs on pc as a rock). How you basically do these questions is by first drawing the empty AO and MO, then counting ... Learn how to apply molecular orbital theory to determine the shapes of bonded orbitals, recognize molecular orbital diagrams, calculate bond order, and determine relative bond strength. Updated ... The MO diagram can be used to calculate bond order and predict the stability of a species. The MO diagram shows the relative energy and number of electrons in each MO. The MO diagram typically includes valence-shell molecular orbitals only. Re: Bond order and strength. For bond order, I believe you must add up all the bonds attached to one molecule (for example one triple plus one double plus one single) and then divide by the total number of bond groups (3 in this case) to get the bond order. I also found a site that explains this concept more in-depth.
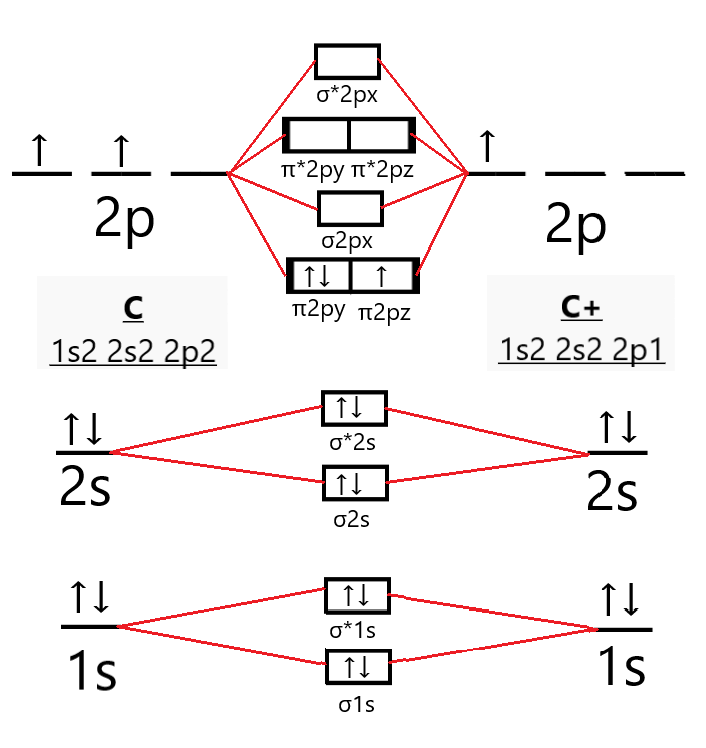
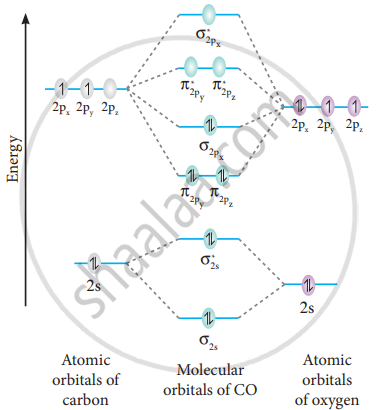
As bond order in Oxygen is 2 so two bonds i.e. double bond is formed between two oxygen atoms (O=O). Further more as there are two unpaired electrons in Oxygen molecule hence it is paramagnetic. Also Watch Molecular orbital diagram of O2 , O2 +2, 02 - 2 ( in Urdu / Hindi) Simplest Trick to Calculate Bond Order : Draw MO diagram of CO and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 17, 2020 by Maisa (45.7k points) selected Dec 18, 2020 by Panna01 . Best answer. 1. Electronic configuration of C atom: 1s 2 2s 2 2p 2. ... From this we can easily draw the Lewis dot diagram of CO2 by adjusting two double bonds between carbon and oxygen (O=C=O). The molecular geometry of CO2 is linear with a bond angle of 180 ° because the dipole charges are canceled by each other as molecule is symmetrically arranged. Although both C=O bonds are polar but the entire molecule is ... How to calculate bond length, stability of molecules, bond order using molecular orbital theory, and bond in homonuclear diatomic molecules. The Molecular orbital theory is also used to predict the reactivity and properties like UV spectra. FAQs on Molecular Orbital Theory. Q.1. What are the main points of molecular orbital theory?
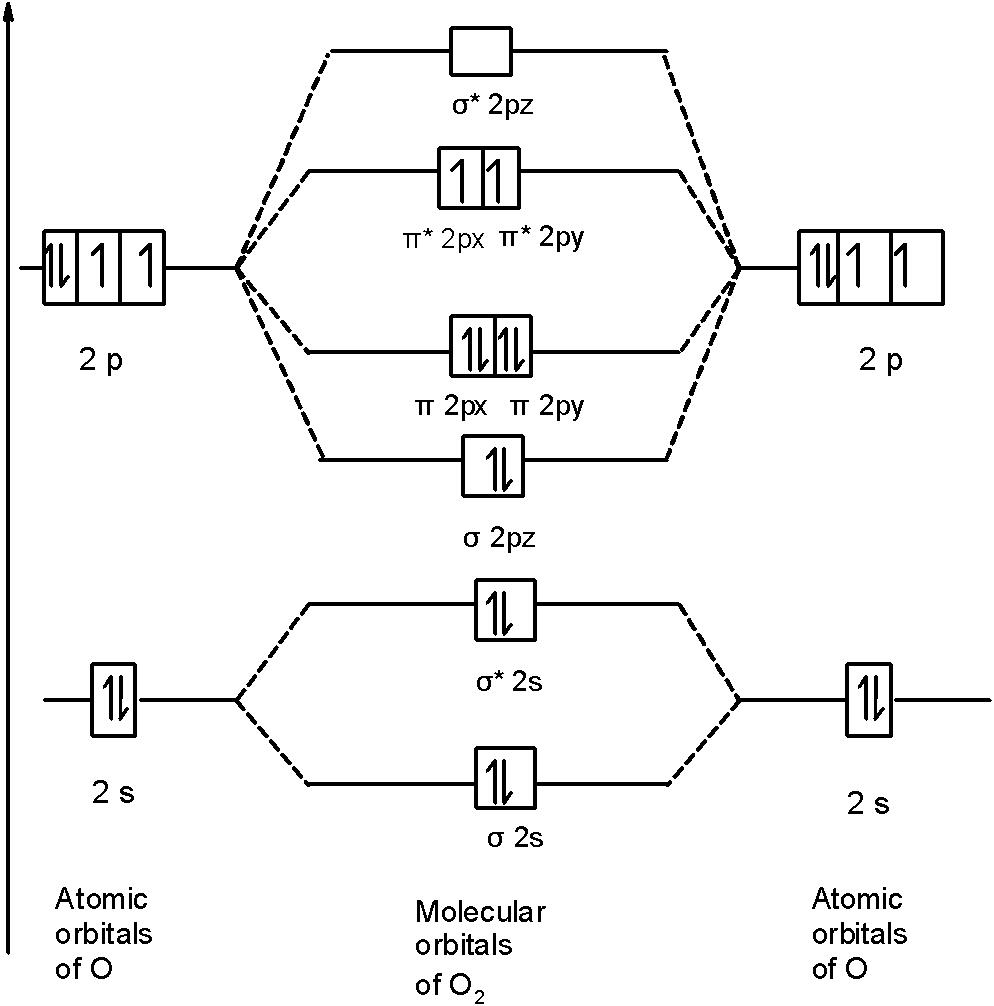
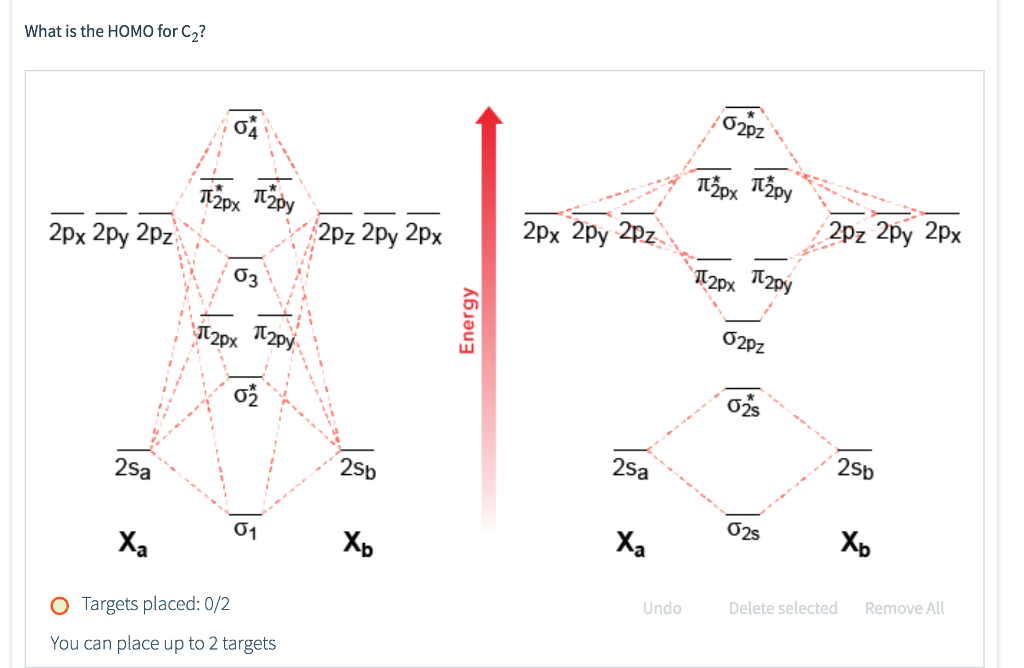
Click here to get an answer to your question ✍️ Draw the molecular orbital diagram of dioxygen and calculate bond order.1 answer · Top answer: Bond order = [ No. of electrons in bonding orbitals ] - [ No. of elevtrons in antibonding orbitals ]2 = 10 - 62 = 42 = 2 Bond order of Oxygen (O) is 2 . O = O Calculation of the bond order of C2(2-) using the MO-diagram. Add Remove. This content was COPIED from BrainMass.com - View the original, and get the already-completed solution here! The problem to solve is to formulate in which way two carbon atoms are described by using atomic orbitals. Thus, when two atoms share two electrons, we depict it by connecting them with two lines. The number of lines, or more precisely, the number of chemical bonds that comprise a molecule, is called its bond order. For instance, the bond order of carbon dioxide and methane is 4, which can easily be discerned by examining their Lewis structures. A MO diagram helps us to find the bonding order of a compound which in result gives us information like bond length, the stability of the compound. Conclusion This article revolves around the structure, bonding, and hybridization of hydronium ions.
Using the MO diagrams shown in Figure \(\PageIndex{11}\), we can add in the electrons and determine the molecular electron configuration and bond order for each of the diatomic molecules. As shown in Table \(\PageIndex{1}\), Be 2 and Ne 2 molecules would have a bond order of 0, and these molecules do not exist.
In molecular orbital theory, bond order is also defined as the difference, divided by two, between the number of bonding and antibonding electrons; ...
Sep 1, 2020 — The number of electrons in an orbital is indicated by a superscript. In this case, the bond order is (1-0)/2=1/2 Because the bond order is ...The Hydrogen Molecule-IonMolecular Orbitals Involvin...Energy-Level Diagrams1 of 3Molecular orbital theory is a conceptual extension of the orbital model, which was so successfully applied to atomic structure. As was once playfully remarked, "a molecule is nothing more than an atom...Continue on chem.libretexts.org »2 of 3We begin our discussion of molecular orbitals with the simplest molecule, H2, formed from two isolated hydrogen atoms, each with a 1s1 electron configuration. As discussed before, electrons can behave...Continue on chem.libretexts.org »3 of 3Because electrons in the σ1s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ1s molecular orbital ha...Continue on chem.libretexts.org »
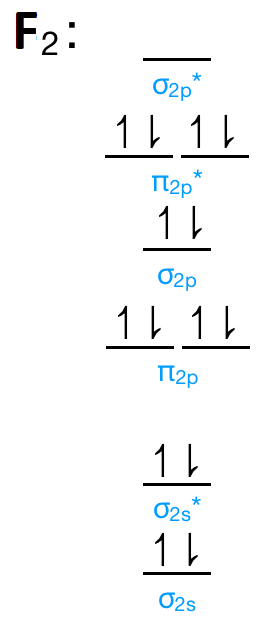
(0.5 marks) (b) Populate the diagram with electrons for F2 (1 mark, redraw the MO diagram yourself if needed) 7 (c) Label the MOs appropriately (1 mark) (d) Identify the HOMO (0.5 marks) (e) Write out the electron configuration for (F2] (0.5 marks) (f) Calculate the bond order for [F21 (0.5 marks) Il + ..
N2O Molecular Orbital Diagram. Molecular orbital diagrams say about the mixing of orbitals in a compound. Using a MO diagram, the bond order of a compound can be determined which gives us an idea about bond length, bond stability as well. Nitrous oxide's MO can be drawn easily by understanding the basics.
A complete molecular orbital diagram would show whether the molecule is diamagnetic or paramagnetic. It can also be used to calculate the bond order of the molecule (the number of bonds between atoms) using the formula below:
Jan 20, 2020 — Bond order formula - molecular orbital theory · Block s: the number of electrons in the valence shell is equal to the element's group (except ...
The XeF4 or Xenon Tetrafluoride is a chemical compound made of Xenon and Fluoride atoms. It is the world's first binary compound discovered. It is a type of noble gas having the chemical equation of. Xe +2 F2 -> XeF4. The XeF4 has a solid white appearance and has a density of 4.040 g cm−3 in a solid form.
In the article Bond order formula, you have grasped the formulas to find the bond order based on Molecular Orbital Theory and Lewis structure. You can explain the information conveyed by the bond order, such as stability, number of bonds, etc.
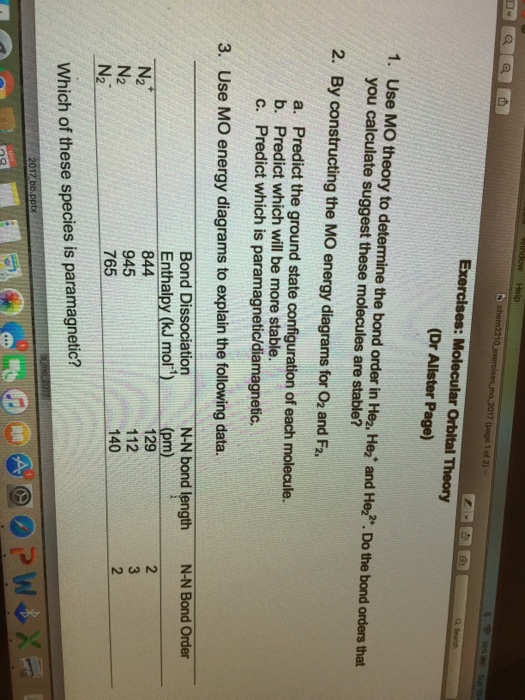
What is the bond order for N 2? The MO method for N2+ gives the bond order equal to 2.5. But first, we look at the diagram of molecular orbitals for N2 (the bond order for the nitrogen molecule is 3).
Describe traits of bonding and antibonding molecular orbitals; Calculate bond orders based on molecular electron configurations; Write molecular electron ...
Re: Bond length. The length of the bond can be determined by the number of bonded electrons (i.e. the bond order). The higher the bond order, the stronger the pull between the two atoms and the shorter the bond length. The length of the bond between two atoms can also be thought of as the sum of the covalent radii of the two atoms.
As bond order in Nitrogen is 3 so three bonds i.e. triple bond is formed between two nitrogen atoms (N≡N). Further more as there is no unpaired electron in Nitrogen molecule hence it is diamagnetic. Simplest Trick to Calculate Bond Order :
Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic. ... It has two unpaired electron in its bonding molecular orbital. ... Draw the M.O diagram for oxygen molecule and calculate its bond order and show that O2 is paramagnetic? asked Oct 1, 2020 in Chemistry by Ruksar02 ...
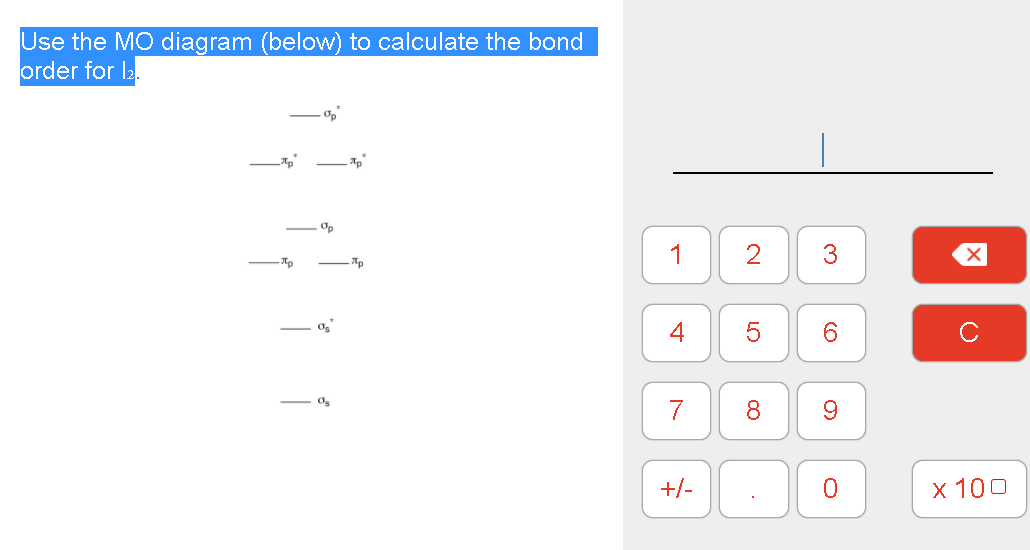
Apps G Gmail Screen Shot 2020-12...2.06 PM Question 17 of 38 Submit Screen Shot 2055 teenshoPM 220200411.994 PPM Use the MO diagram (below) to calculate the bond order for CO. Op Op ло Mp 1 Question : Chrome File Edit View History Bookmarks People Tab Window Help 53% O Sat Dec 12 2:02 PM Q E Dashboard х 101 Chem101 х C Search Textbook ...
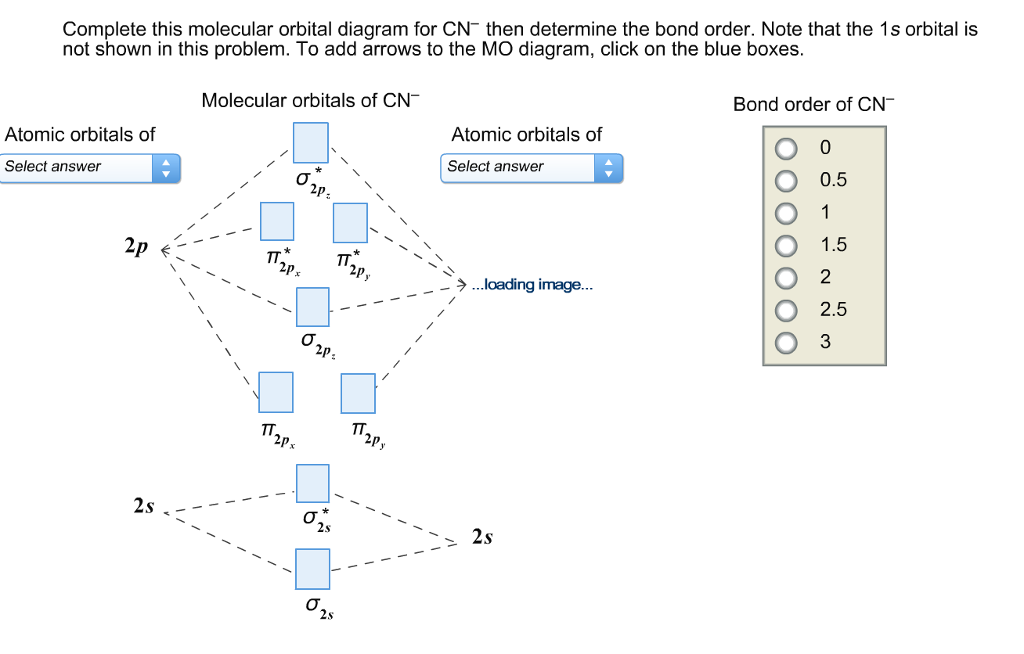
Bond Order Formula. The following formula is used to calculate a bond order. BO = ( EA- EB) / 2. Where BO is the bond order. EA is the number of electrons in the antibonding MO. EB is the number of electrons in the bonding MO.
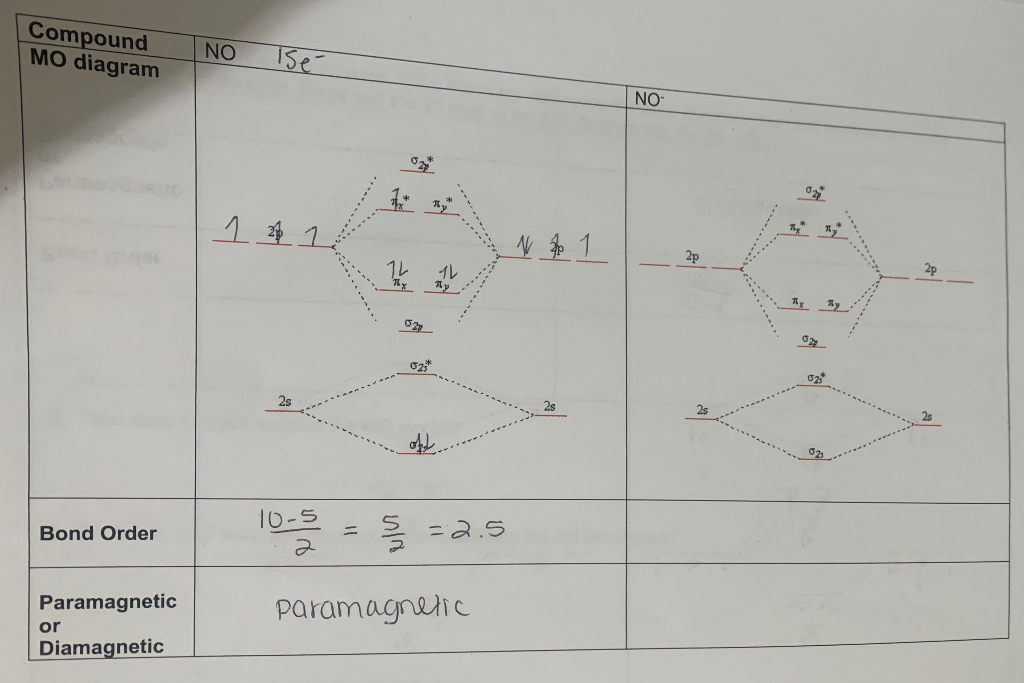
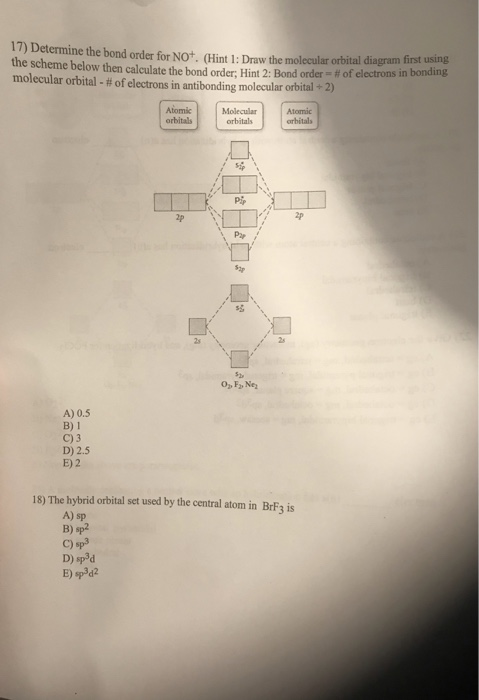
Aug 29, 2017 · 1 answerThe MO diagram for NO is as follows (Miessler et al., Answer Key):. (The original was this; I added the orbital depictions and symmetry ...
The lesser the bond order, the greater is the bond length as the bond order is inversely proportional to bond length. ( Hence O 2 2- has a larger bond length than O 2 2- . Chemical Bonding and Molecular Structure Important Extra Questions Long Answer Type
In molecular orbital theory, bond order is also defined as half of the difference between the number of bonding and antibonding electrons.Dec 5, 2017 · Uploaded by chemistNATE
The double bond of butane is composed of a π bond formed by the overlap of p orbitals and a σ bond formed by the overlap of sp2 hybrid orbitals. A 90-degree rotation about the bond eliminates all overlap of the p orbitals that form the π bond and it is broken.
We use molecular orbital theory to calculate the bond order between two atoms. It's very interesting to draw the energy level diagram of the atoms. Read more What is the Bond Order in F2? Answer- 1 is the bond order for an f2 molecule. We calculate the bond order with the help of molecular orbital theory or bond order theory.




















0 Response to "40 calculate bond order from mo diagram"
Post a Comment