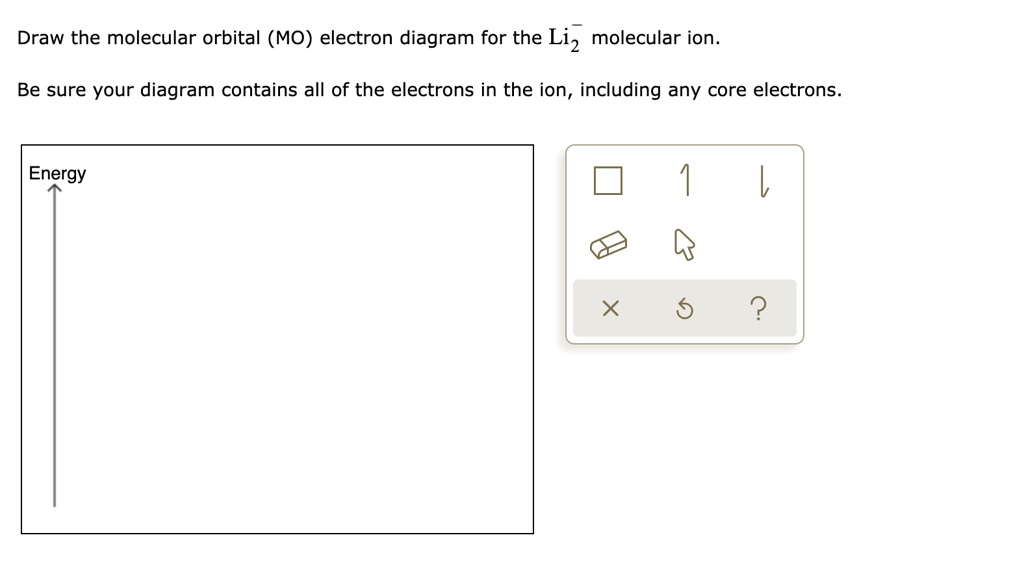
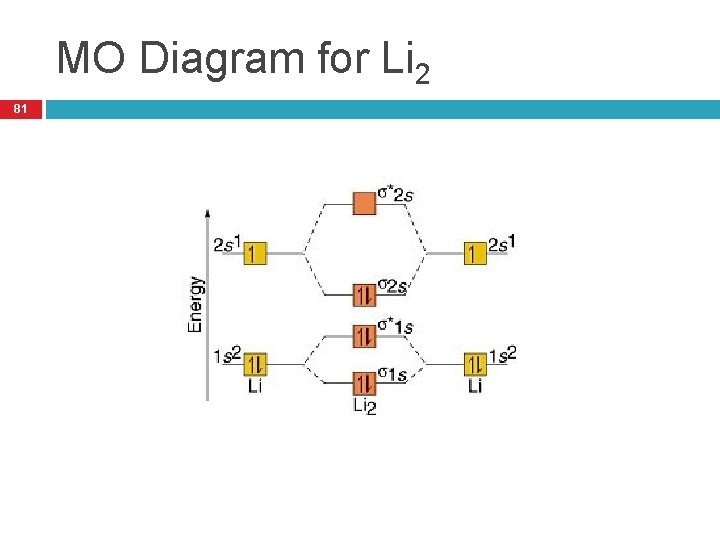
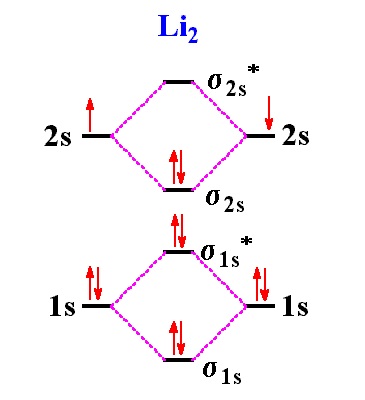
40 mo diagram for li2
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine PLEASE. My friend Mo Diagram For Li2 And Be2 Dating do not try to use money to get sex from women. There is a 37 year old man who has Mo Diagram For Li2 And Be2 Dating a child, and describes himself as Mo Diagram For Li2 And Be2 Dating an old fat balding ginger. He gets casual sex from many different attractive women on a regular basis. His name is Owen Cook (he also goes by the name of tyler ...
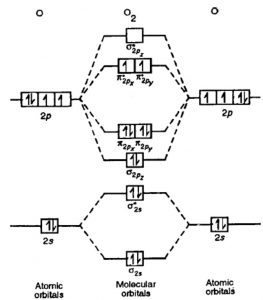
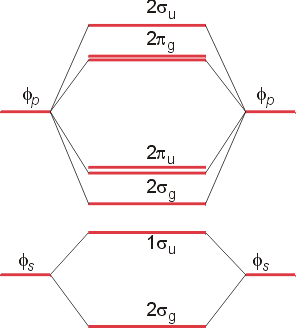
The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules. The same method can be applied to other diatomic molecules, but involving more than the 1s atomic orbitals. For the second period elements, the 2s and 2p orbitals are important for MO considerations. A linear combination of properly oriented atomic orbitals for the formation of sigma s and pi p bonds.
Mo diagram for li2
This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o... 1)Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li2+ and Li2-. Best Answer. This is the best answer based on feedback and ratings. 89% (18 ratings) Get answers by asking now. The molecule Li2 is a stable molecule in the gas phase, with a bond order of one.Molecular orbital diagram - WikipediaWhat is the bonding order of Li2? Molecular Orbital Theory (M.O.T.) Use the drawing of the MO energy diagram to predict the bond order of Li2?. The electron configuration of Li 2 may be written as σ 2..
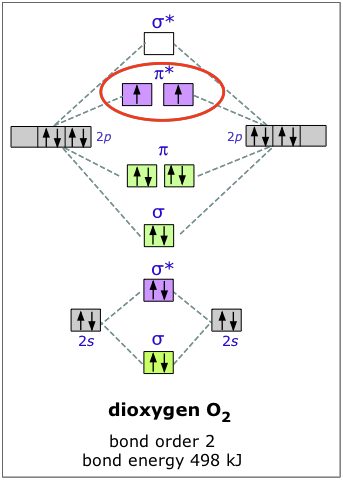
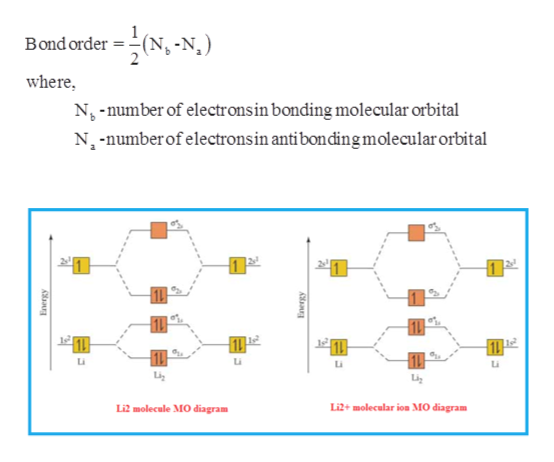
Mo diagram for li2. Since the bond order of Li 2 is higher than Li 2 + and Li 2 - . Therefore ,Li2 has stronger single covalent bonding . Thus lithium molecule (Li2) is more stable. Li 2 + is more stable as compared to Li 2 - :. Li 2 + and Li 2 - ions have the same bond order ( 0.5) .. But Li 2 - has more electrons in higher energy antibonding molecular orbital as compared to Li 2 + . Molecular orbital diagrams for li2 li2 be2 b2 c2 n2 chemistry enthusiasts by megha khandelwal. For the second period elements the 2 s and 2 p orbitals are important for mo considerations. This is peroxide ion. Determine the magnetism of simple molecules. The same method can be applied to other diatomic molecules but involving more than the 1 s ... Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = (). B.O = 1. Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. Answer to Draw a molecular ... The following MO diagram is appropriate for Li2 and Be2. Based on this diagram, Li2 is stable and diamagnetic, but Be2 is unstable. Given that O2 is paramagnetic and has a bond order of 2, and its highest occupied molecular orbital is antibonding, what would be the expected bond orders for O22- and O22+?
Answer (1 of 5): Bond order is the number of chemical bonds between a pair of atoms. Bond order is the number of chemical bonds between a pair of atoms; in diatomic nitrogen (N≡N) for example, the bond order is 3, while in acetylene (H−C≡C−H), the bond order between the two carbon atoms is 3 and... The valence molecular orbital diagram for Li2- is shown. The molecular orbital bond order for this species is equal to _____ and Li2- is _____ stable than Li2.-1/2, less. Which of the following statements correctly describe the rules used for placing electrons in molecular orbitals? Select all that apply. #3. Draw the MO diagram for `O_2^+` This is a bit of a curveball, but a perfectly valid problem. Recall that a cation indicates a loss of `1` electron. `O_2^+` is just the ionized form of `O_2`; that is, it's `O_2` with `1` missing electron. The MO diagram will be the same as the MO diagram of `O_2`, except with `1` less electron. Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and. for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding ... MO diagram for Li2. Moderators: Chem_Mod, Chem_Admin. 2 posts • Page 1 of 1. Chem_Mod Posts: 19945 Joined: Thu Aug 04, 2011 8:53 pm Has upvoted: 999 times. MO diagram for Li2. Post by Chem_Mod » Mon Nov 21, 2011 11:21 pm . Question: For molecules like Li2 where the p MO's aren't used and thus have no electrons, do we still have to draw them ... Use the drawing of mo energy diagram for co to predict the bond order. The bond order for be2 is calculated by. Draw the energy lebel diagram for o 2 2 with help of pen and paper and sho. Bond order indicates the stability of a bond. Answer to draw an mo energy diagram and predict the bond order of li2 and li2. C would this ion exist. Part b complete the ao and mo energy diagram for li2 drag the appropriate labels to their respective targets. The bond order for li2 is 1. A molecular orbital mo energy level diagram appropriate for homonuclear diatomic molecules from li2 to n2 is shown below. The mo bond order is the main factor controlling the internucelar distance.
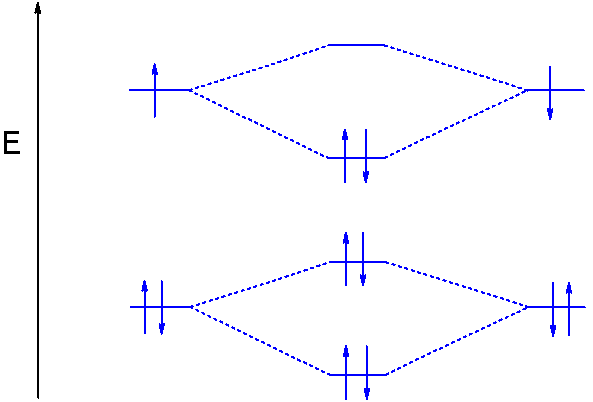
Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital.
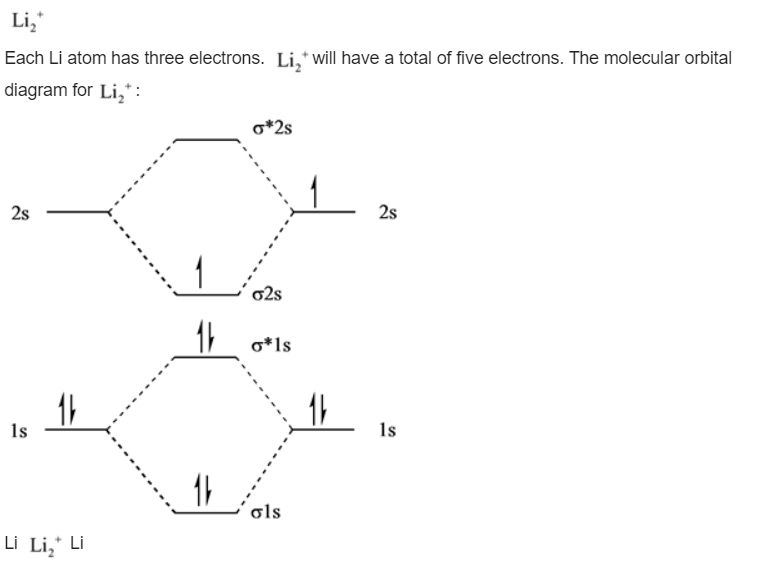
Answer: Molecular orbital electronic configuration of Li₂+ : σ1s² σ*1s²σ2s¹. So, bond order = (Nb - Na)/2 = (3-2)/2 = 0.5. Molecular orbital electronic configuration of Li₂- : σ1s² σ*1s²σ2s2 σ*2s¹, bond order = (Nb - Na)/2 = (4-3)/2 = 0.5. As the bond orders are same in both the species, it is ...
How to draw Molecular Orbital Diagram of Li2 ,Li 2+ , Li2 - | Simplest Trick - Chemistry By anumsunum on August 20, 2021 • ( Leave a comment ) Also Watch Molecular orbital diagram of O2 , O2 +2 , 02 - 2 ( in Urdu / Hindi)
As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear combinations such as CO and NO. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2.
Molecular orbital energy level of Li2. MO diagrams for Diatomic Molcules. Overview. In this section, we will compare MO diagrams for diatomic molecules X-X, from Li2 to Ne2. Molecular orbital diagram of Li2 & Be2: Number of electrons in Li2 molecule =6. Li2 = σ1s2,σ*1s2,σ2s2. Nb=4, Na=2. B.O = (Nb- Na). B.O = ().
The molecule Li2 is a stable molecule in the gas phase, with a bond order of one. Bond Order=2(bonding electrons)−0(anti−bonding e−)2=1. Subsequently, question is, is li2 stable or unstable? Li2+ is more stable than Li2− because Li2− has more numbers of antibonding electrons. The species in which the N atom is in a state of sp ...
This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
Molecular orbital diagrams of diatomic molecules. 1complete the atomic orbital ao and molecular orbital mo energy diagram for li2 and li2 1complete the atomic orbital ao and molecular orbital mo energy diagram for li2 and li2 best answer. If the phase changes the bond becomes a pi bond π bond. Bond order is 3 and it is paramagnetic.
Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
Full first shell:sigma-1s and sigma1s* are both full.sigma-2s is full; sigma-2s* is empty.Bond order 1 = single bond.Check me out: http://www.chemistnate.com
Get answers by asking now. The molecule Li2 is a stable molecule in the gas phase, with a bond order of one.Molecular orbital diagram - WikipediaWhat is the bonding order of Li2? Molecular Orbital Theory (M.O.T.) Use the drawing of the MO energy diagram to predict the bond order of Li2?. The electron configuration of Li 2 may be written as σ 2..
1)Complete the atomic orbital (AO) and molecular orbital (MO) energy diagram for Li2+ and Li2-. Best Answer. This is the best answer based on feedback and ratings. 89% (18 ratings)
This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o...


















0 Response to "40 mo diagram for li2"
Post a Comment