42 Transition States Energy Diagram
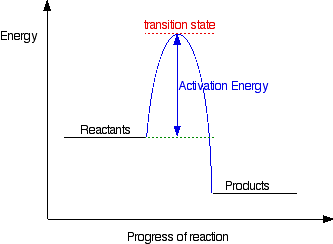
energy profiles - chemguide The second diagram where the bonds are half-made and half-broken is called the transition state, and it is at this point that the energy of the system is at its maximum.This is what is at the top of the activation energy barrier. But the transition state is entirely unstable. Jablonski Energy Diagram - Florida State University Both of the triplet state transitions are diagrammed on the right-hand side of the Jablonski energy profile illustrated in Figure 1. The low probability of intersystem crossing arises from the fact that molecules must first undergo spin conversion to produce unpaired electrons, an unfavorable process.
Exothermic Energy Diagram: Activation Energy, Transition ... Exothermic Energy Diagram: Activation Energy, Transition States and Enthalpy Change - TUTOR HOTLINE - YouTube. Exothermic Energy Diagram: Activation Energy, Transition States and Enthalpy Change ...
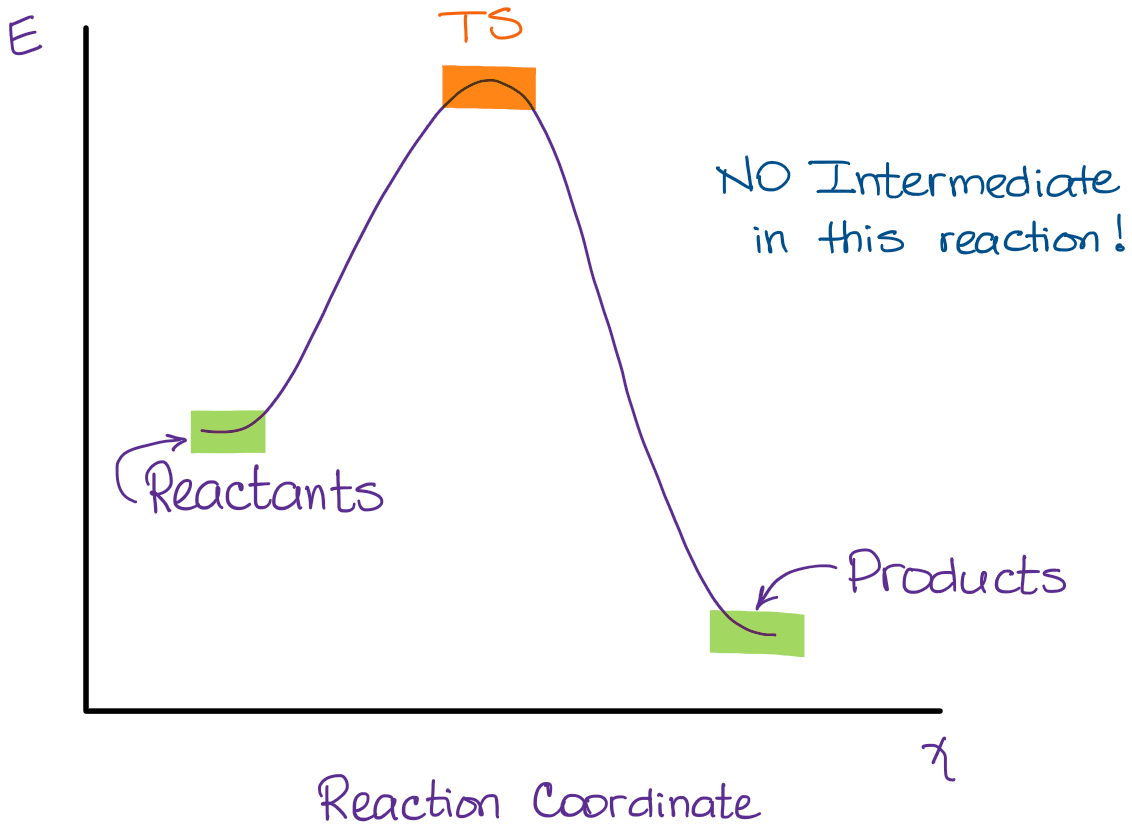
Transition states energy diagram
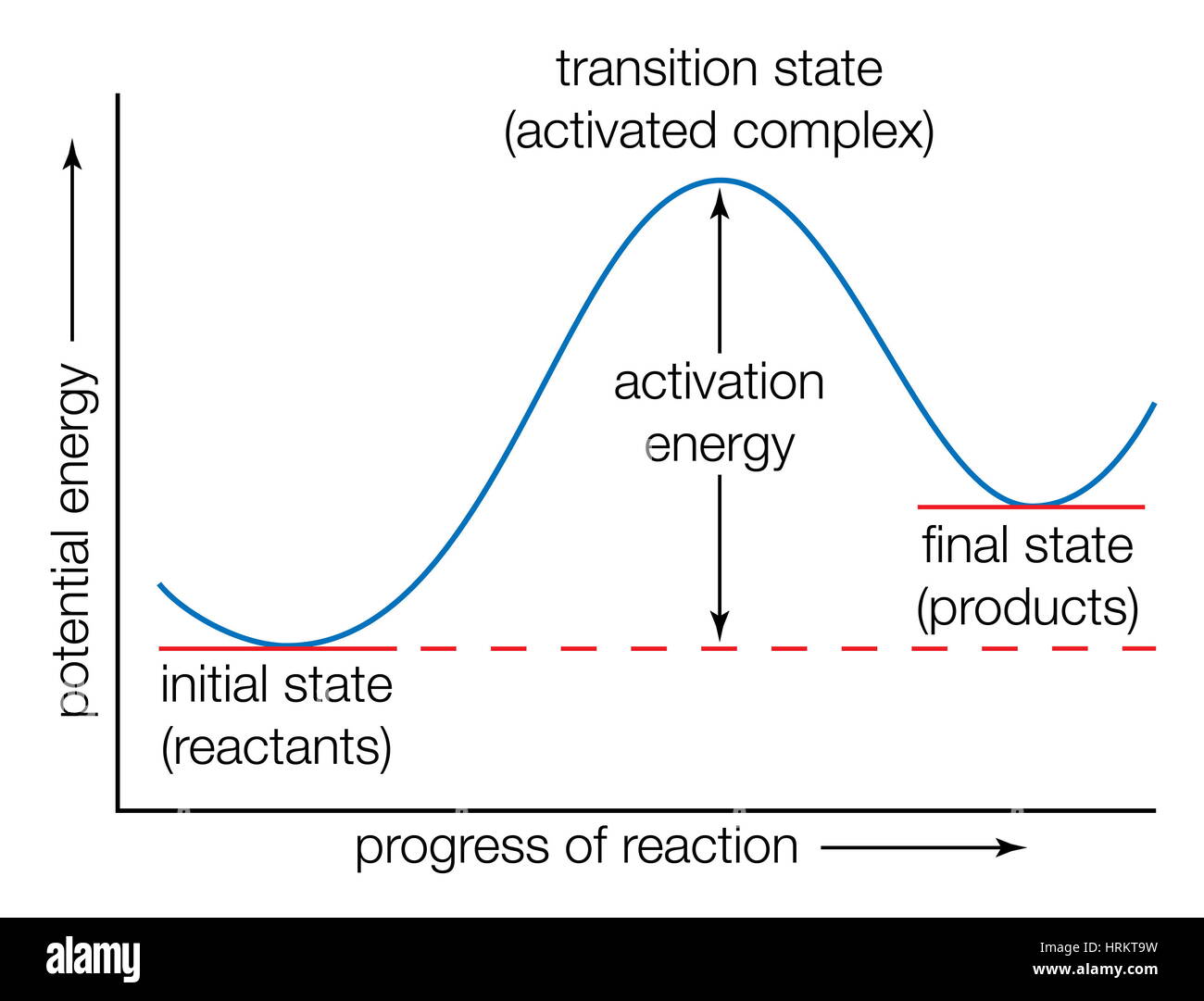
PDF Teacher's Guide to "Visualizing the Transition State and ... Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a transition state to products (see figure 1). The reaction energy diagram plots the Energy Diagrams: Describing Chemical Reactions Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (Ea), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1. Arrhenius Theory and Reaction Coordinates The transition state is the higher energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state. The transition states are often very difficult to identify and during a reaction the molecules exist in this state for essentially zero time.
Transition states energy diagram. Learn About Transition State In Energy Diagram | Chegg.com Transition State in Energy Diagram Transition State in Energy Diagram Definition An energy diagram refers to the plot which shows the reactants' relative potential energies, its transition states, as well as the products progression of the reaction with time. It is a plot between potential energy and reaction progress or time. en.wikipedia.org › wiki › Morse_potentialMorse potential - Wikipedia The Morse potential, named after physicist Philip M. Morse, is a convenient interatomic interaction model for the potential energy of a diatomic molecule.It is a better approximation for the vibrational structure of the molecule than the quantum harmonic oscillator because it explicitly includes the effects of bond breaking, such as the existence of unbound states. Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... What is the Difference Between a Transition State and an ... Transition state is the highest point (or points) on the reaction coordinate diagram. Those are the "peaks" or the "hills" in the picture. A more strict definition is that a transition state is a molecular entity that has a lifetime no longer than a vibration that exhibits some structural characteristics of both the reactants and the products .
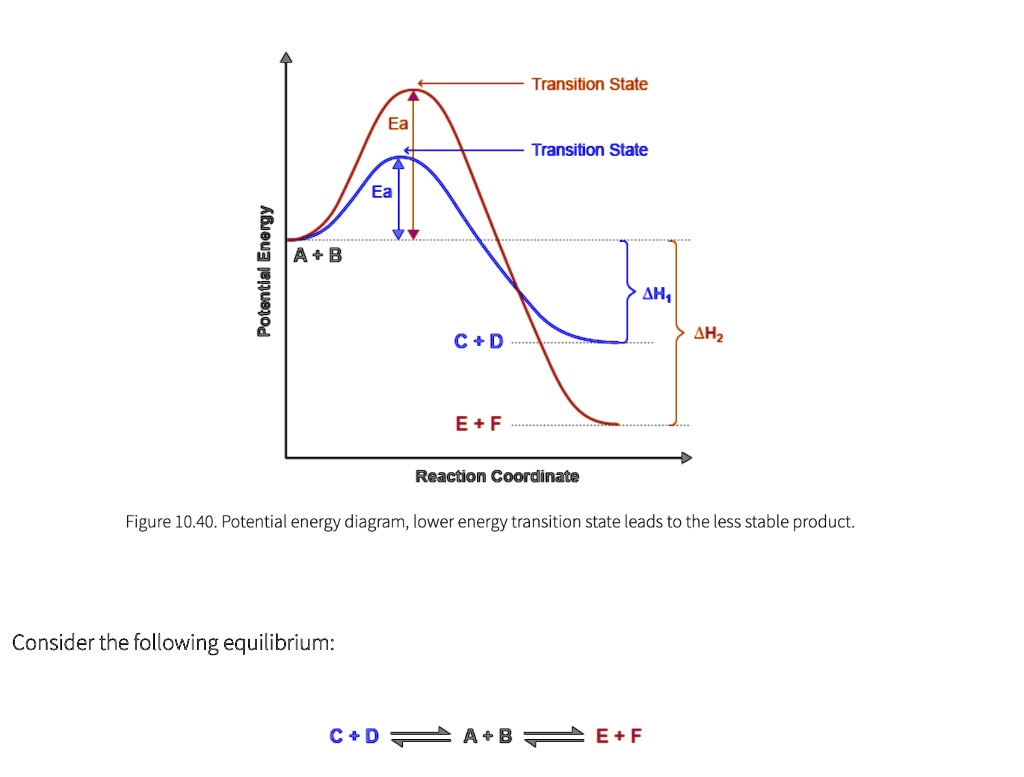
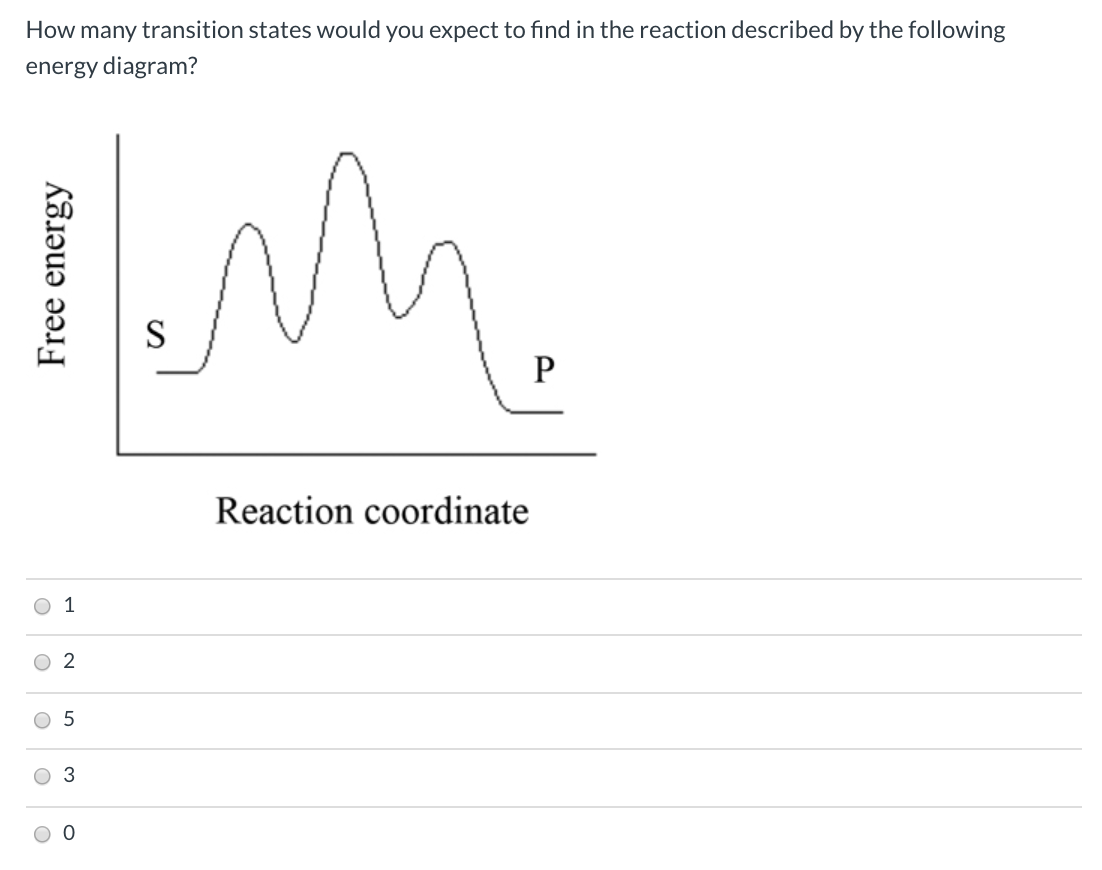
Solved How many intermediates and transition states are in ... How many intermediates and transition states are in the mechanism represented by the following free energy diagram? Question : How many intermediates and transition states are in the mechanism represented by the following free energy diagram? Jablonski Diagram | What is it? | Edinburgh Instruments The energy of the photon is converted to the internal energy of the molecule. A molecule is promoted from its ground state to a higher state by absorption of a photon which is represented by the blue arrows in Figure 2. It is the fastest transition in the Jablonski diagram, occurring on a timescale of order 10-15 s. At room temperature, the ... › articles › 415039aQuantum phase transition from a superfluid to a Mott ... Jan 03, 2002 · We can induce reversible changes between the two ground states of the system. ... of a quantum phase transition is that this energy gap ... basis of the superfluid–Mott insulator phase diagram. › phase-transitionPhase Transition - an overview | ScienceDirect Topics A first-order phase transition is characterized by a discontinuous jump in the first derivative of the Gibbs free energy (G) in function of the temperature (T), ∂G(T)/∂T. In a so-called thermogram , in which the heat flow is plotted as a function of time ( t ) or temperature ( T ), first-order phase transitions can be visually distinguished ...
PDF Page Notifications Off Spectroscopy > Jablonski diagram ... A Jablonski diagram is basically an energy diagram, arranged with energy on a vertical axis. The energy levels can be quantitatively denoted, but most of these diagrams use energy levels schematically. The rest of the diagram is arranged into columns. Every column 5.6: Reaction Energy Diagrams and Transition States ... 5.6: Reaction Energy Diagrams and Transition States draw Reaction Energy Diagrams from the thermodynamic and kinetic data/information use a Reaction Energy Diagram to discuss transition states, Ea, intermediates & rate determining step draw the transition state of a reaction GitHub - ctesvara/Energy_diagram_withTS: Adding curved ... to use, simply add this line: diagram.add_ts(initial_ID, final_ID, TS_energy) example: diagram.add_ts(0, 2, -1.5) About Adding curved line to describe a Transition State in energy_diagram.py Transition State Theory The entire energy diagram for the ABC system is shown in 3-D in Figure PRS.3B-3. To obtain Figure PRS.3B-2 from Figure PRS.3B-3 we start from the initial state (A + BC) and move through the valley up over the barrier, E b, (which is also in a valley) over to the valley on the other side of the barrier to the final state (A + BC).
Difference between intermediates and transition states A transition state is a chemical species which has only fleeting existence and represents an energy maxima on reaction coordination diagram . While an intermediate lies in depression on potential energy curve . Therefore actual lifetime of an intermediate depends on the depth of the depression.
State-Transition Diagrams - StickyMinds State-Transition Diagrams State-transition diagrams describe all of the states that an object can have, the events under which an object changes state (transitions), the conditions that must be fulfilled before the transition will occur (guards), and the activities undertaken during the life of an object (actions).
PDF Structure of the Transition State the beginning state. The energy of the TS≠ must be closer to the beginning state. There's just no other way to draw the diagram. We call this TS≠ "early" since the structure of the transition state has not evolved far from its starting point. Case 2: the ending state is higher than the beginning state - In this case
www2.lbl.gov › abc › wallchartChapter 6 Nuclear Energy Levels - Lawrence Berkeley National ... Fig. 6-1. Energy level diagram of some of the excited states of the 12C nucleus. The angular momentum (J), parity (P), and isospin (T) quantum numbers of the states are indicated on the left using the notation J P. P and n respectively at the top of the diagram indicate the separation energies for a proton and a neutron.
Energy Diagram Module Series- Part Three: Intermediates ... Posted on August 12th, 2013. This is part 3 of a four part series in the Energy Diagram Module. Stay tuned for Part 4! Click on the following links to see earlier parts: Part 1. Part 2. Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism.
Transition State Theory - Concept, Formation, Formula and ... The transition state is defined as the attained by the activated complex configuration along with the reaction coordinate where the potential energy of the reaction is highest as compared to the reactants and the final product formed at the compilation of the reaction whereas the activated complex is the unstable complex formed at the intermediate coordinate of the reaction where the potential energy is the highest.
State Transition Diagram - an overview | ScienceDirect Topics The state transition diagram as shown in Figure 8.6 illustrates the active and quiescent states that are supported by the logic and the paths between these states. The state transition diagram also illustrates the states and transitions of the communication protocol between the recipe phase and the equipment phase. The phase logic must adhere to the rules depicted in the state transition diagram. Only valid state transitions as depicted in Figure 8.6 may be utilized. Though the configuration ...
Energy Diagrams, Transition States, and Reactive ... Peaks on the energy diagram represent the transition states, whereas the valleys represent the reactive intermediates. As the reaction progresses, the reactants pass through an unstable state of maximum free energy, called the activated complex, or the transition state. They last for less than one picosecond and cannot be isolated.
Energy Level and Transition of Electrons | Brilliant Math ... Since a longer wavelength means smaller energy, the red line correspond to the transition which emits the lowest energy within the Balmer series, which is n = 3 → n = 2. n=3\rightarrow n=2. n = 3 → n = 2. The turquoise line indicates the transition with the second lowest energy within the Balmer series, which is n = 4 → n = 2. n=4\rightarrow n=2. n = 4 → n = 2.
PDF Orgel and Tanabe-Sugano Diagrams for Transition Metal ... Orgel diagrams are the oversimplified version of correlation diagrams that show the relative energies of electronic terms in transition metal complexes. They are named after their inventor, Leslie Orgel. These diagrams are restricted only to show weak field cases and offer no information about strong field cases. Because Orgel diagrams are qualitative, no energy calculations can be performed from these diagrams.
Energy profile (chemistry) - Wikipedia The saddle point represents the highest energy point lying on the reaction coordinate connecting the reactant and product; this is known as the transition state. A reaction coordinate diagram may also have one or more transient intermediates which are shown by high energy wells connected via a transition state peak.
6.9: Describing a Reaction - Energy Diagrams and ... 6.9: Describing a Reaction - Energy Diagrams and Transition States sketch the reaction energy diagram for a single-step reaction, given some indication of whether the reaction is fast or... interpret the reaction energy diagram for a single-step process (e.g., use the diagram to decide whether the ...
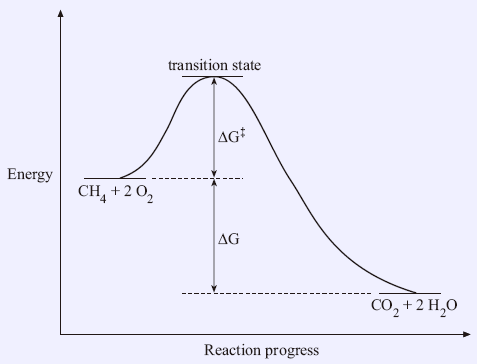
Transition states and activation energy | Open Textbooks ... A reaction starting from reactants that change into products must pass through an unstable state at the highest energy level; this is called a transition state. A certain amount of energy is required to overcome the energy barrier, and this energy, called activation energy, is represented by ΔG ‡. The magnitude of ΔG ‡ determines the rate of a chemical reaction. When ΔG ‡ increases, the reaction
CH 368: Unit 2 - University of Texas at Austin Definition of Transition State. The transition state of a reaction step is considered to be the highest energy point on the minimum (free) energy reaction path (MERP). Reactions are considered to occur via the path from reactants to products which is of lowest energy. The TS is the point of highest energy on this path.
Arrhenius Theory and Reaction Coordinates The transition state is the higher energy point between two minima along the reaction coordinate. Each step in a mechanism will have a transition state. The transition states are often very difficult to identify and during a reaction the molecules exist in this state for essentially zero time.
Energy Diagrams: Describing Chemical Reactions Energy diagrams are quite useful in illustrating these changes on a continuous basis as the reaction proceeds. Terms such as "activation energy" (Ea), "transition state" (*), and "enthalpy change" are easy to define by referring to a graph such as Figure 1.
PDF Teacher's Guide to "Visualizing the Transition State and ... Transition state theory (TST), also called activated complex theory, is often introduced in general chemistry courses when discussing kinetics. A reaction energy diagram is used to follow the progress of the reaction from reactants through a transition state to products (see figure 1). The reaction energy diagram plots the












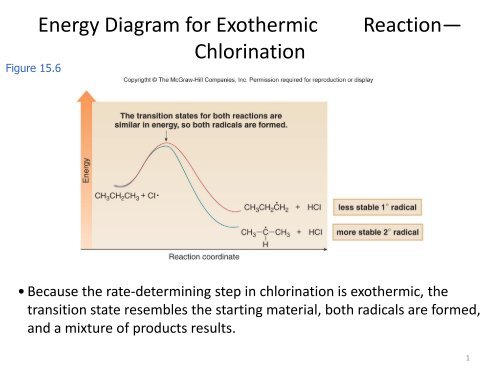


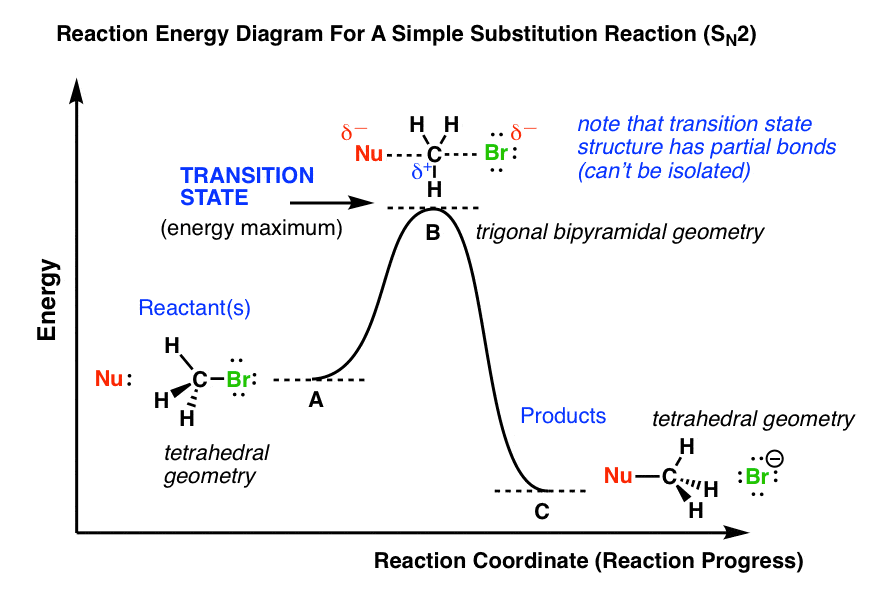
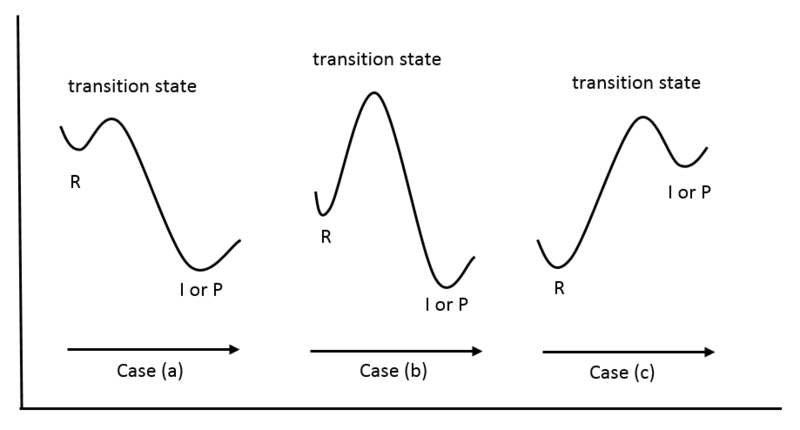






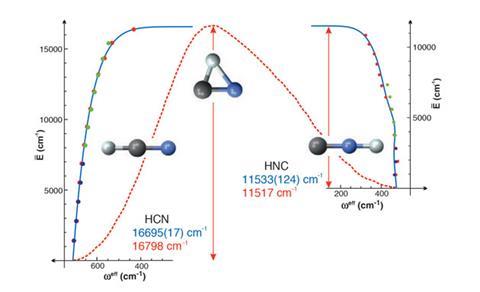




0 Response to "42 Transition States Energy Diagram"
Post a Comment