42 Energy Diagram For Endothermic Reaction
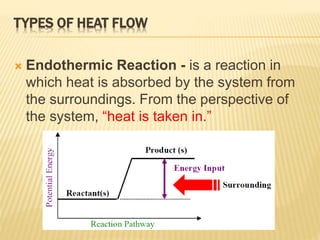
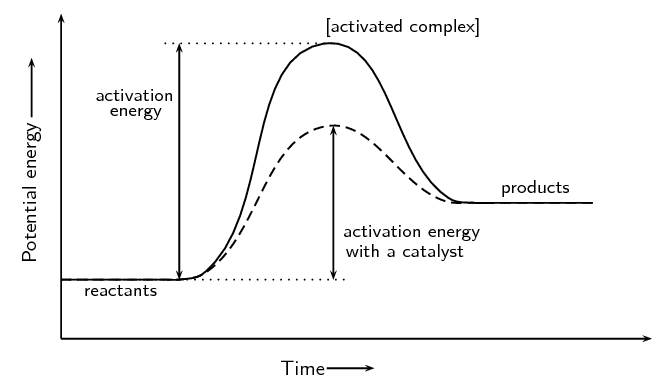
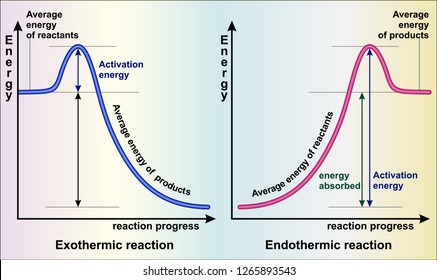
Endothermic Reactions: Definition, Example, Diagram and ... Sep 02, 2021 · Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. Potential Energy Diagrams - Kentchemistry.com Both endothermic and exothermic reactions require activation energy. Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products.
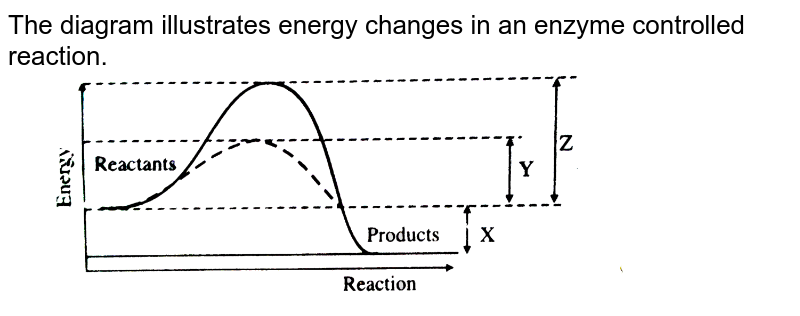
Energy Diagrams | OpenOChem Learn An exergonic reaction has ΔG < 0 and occur spontaneously, while an endergonic reaction has ΔG > 0 Recall that ΔG is the Gibbs free energy and is defined as follows ΔG = ΔH - TΔS. Multi-Step Reactions The reactions energy diagrams shown above are for single step reactions. In a single reaction step there is only one barrier (hump) in the diagram.
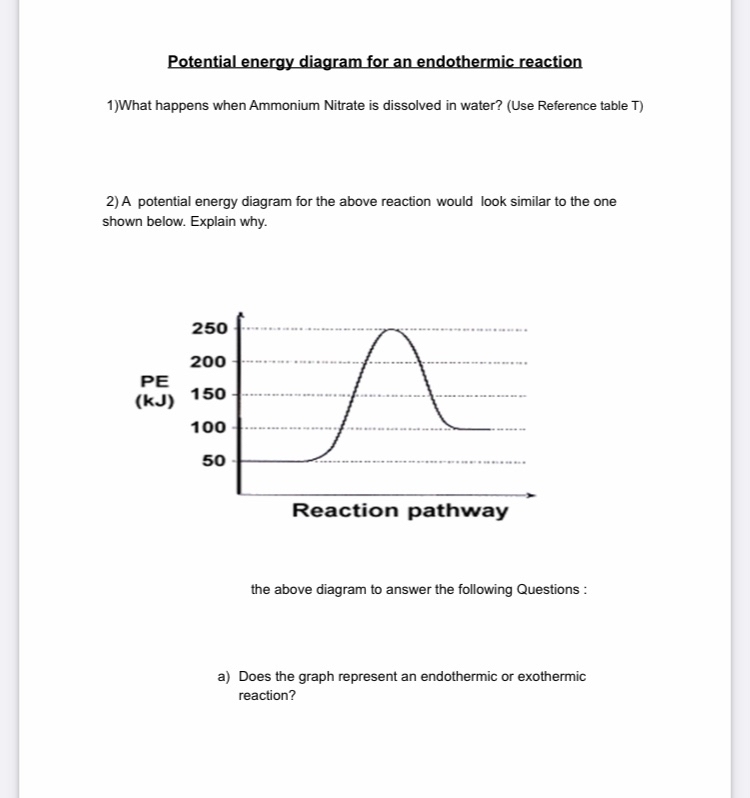
Energy diagram for endothermic reaction
Reaction Coordinate Diagrams The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction. Energy Diagrams of Reactions | Fiveable Nov 23, 2021 · Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings. Potential Energy Diagrams - Chemistry - Catalyst, Endothermic ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
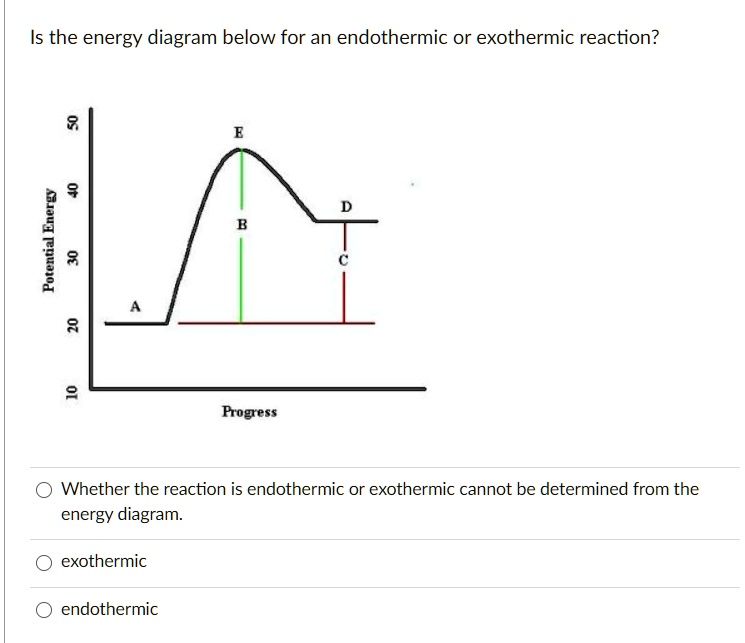
Energy diagram for endothermic reaction. Endothermic Reaction | Characteristics, Examples ... Energy level diagram for an Endothermic change. Thermal energy from the Surroundings is absorbed by the System. This results in a higher Enthalpy value of the final state with respect to the... Reaction profiles - Exothermic and endothermic reactions - AQA An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The... Endothermic vs. exothermic reactions (article) | Khan Academy Energy diagrams for endothermic and exothermic reactions In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. In other words, the products are less stable than the reactants. Solved Question 15 (7 points) Listen Sketch the potential ... Question 15 (7 points) Listen Sketch the potential energy diagram for an endothermic reaction. On the diagram, mark the location of the activation energy, transition state reactants, and products. (Note: this will probably be easiest to submit through your handwritten work only. You can type "see handwritten work" below if needed.) Question ...
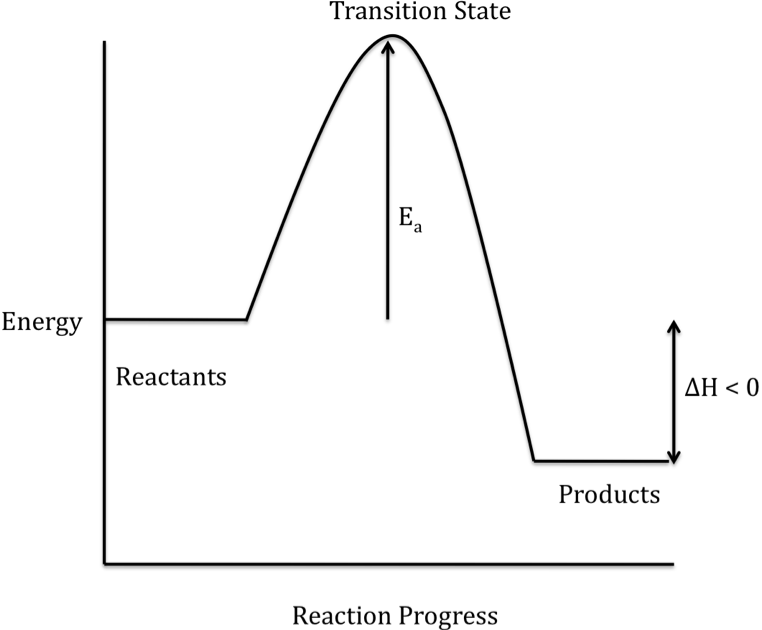
Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk. Energy level diagrams - Why are there energy changes in ... Energy level diagrams are used to model energy changes during reactions. They show the relative energy levels of the products and reactants. Exothermic reaction The energy level decreases in an... What are Endothermic Reactions? (with Examples & Video) Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is generally lower than that of the reactant. PDF Exothermic vs endothermic reaction graphs State one reason, in terms of energy, to support your answer.Answer-->Endothermic, the products have more energy than the reactants.b) On the diagram provided in your answer booklet, draw a dashed line to indicate a potential energy curve for the reaction if a catalyst is added.Answer--
Energy in Chemical Reactions I Unit Test Flashcards | Quizlet It is an endothermic reaction because the system takes energy from the surroundings. A student pours ammonium nitrate into a container of room temperature water. The student stirs the mixture with a stirring rod and then feels the outside of the container, which is cold to the touch. How can I represent an endothermic reaction in a potential ... An endothermic reaction must have a positive change in enthalpy. That is, Delta"H">0. This means that the system absorbs heat. To represent the positive change in enthalpy, the potential energy of the reactants must be LOWER than that of the products. Notice how the end of the "hump" is higher than the beginning, showing how the system gained energy during the course of the reaction. Energy Profiles (Energy Diagrams) Chemistry Tutorial Energy Profile for Endothermic Reactions We saw above that the synthesis of ammonia gas from nitrogen gas and hydrogen gas was an exothermic process: N 2 (g) + 3H 2 (g) → 2NH 3 (g) + 92.4 kJ mol -1 and we constructed an Energy Profile to show the relative enthalpies of reactants and products. 18.4: Potential Energy Diagrams - Chemistry LibreTexts A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change ( Δ H) is positive for an endothermic reaction and negative for an exothermic reaction.
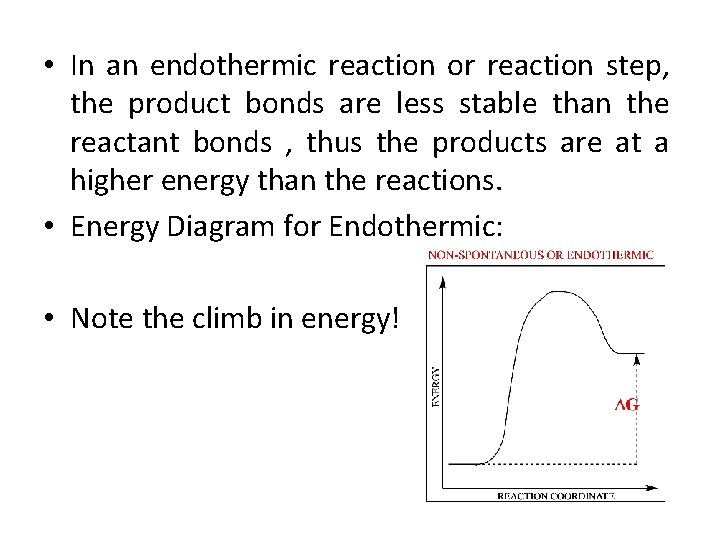
7.3: Exothermic and Endothermic Reactions - Chemistry ... Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure 7.3. 2. In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds. Hence, the energy of the reactants is lower than that of the products.
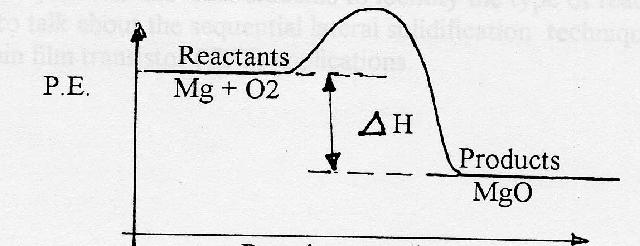
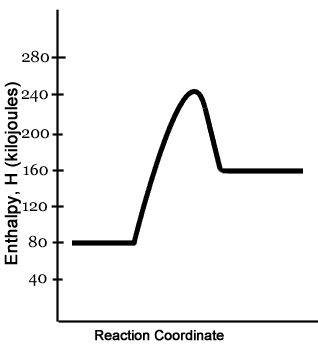
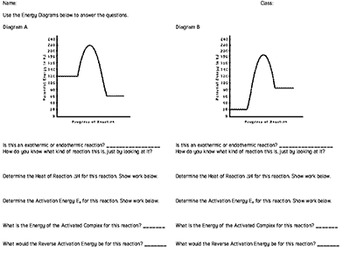
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic.
How does the energy level diagram show this reaction is ... In an endothermic reaction, the reactants absorb heat energy from the surroundings to form products. Thus, the products formed have more energy than the reactans, H products > H reactants. Therefore, ΔH is positive. Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show:
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction. In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. ... In endothermic reactions, there is less energy in the reactants than ...
Copy of 7.01 Alternate Assignment 2022.pdf - 7.01 ... 19) How do potential energy diagrams differ between endothermic and exothermic reactions? In an endothermic diagram it has a lower enthalpy at the end while in the exothermic diagram the products are higher and have a higher enthalpy. 20) Due to the increase in enthalpy between reactants and products, endothermic reactions have a ____high ...
Solved The reaction energy diagrams for an endothermic and ... The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of ...
PPTX Energy and Chemical Reactions - Boyertown Area School ... Endothermic Diagram. Energy absorbed in reaction. Activation . Energy. Energy used in bond. breaking. Endothermic - more energy is taken in to break the bonds in the reactants than released by the bonds being formed in the products. Therefore, energy is absorbed. Energy released in bond making
Draw an energy diagram for an endothermic reaction without a ... Explanation: Endothermic reactions are defined as the reactions in which energy of the product is greater than the energy of the reactants.The total energy is absorbed in the form of heat and for the reaction comes out to be positive.. Catalyst is the substance which does not participate in the reaction but increases the rate of the reaction and results in the early formation of product.
Solved 3. Based on the following energy diagram, is the ... Chemistry. Chemistry questions and answers. 3. Based on the following energy diagram, is the reaction exothermic or endothermic? How many steps are in this reaction? How many transition states and intermediates? free energy, kJ/mol reaction coordinate 4. Based on the following energy diagram, is the reaction exothermic or endothermic?
Potential energy diagram questions chemistry pdf ... Graph 1: Use the potential energy diagram for the reaction X + Y -> Z to complete the chart below. Graph 2 1. Draw a potential energy diagram for an endothermic reaction. A compendium of past examination questions set on Physical Chemistry on the JF Chemistry paper and problem sheets associated with CH1101 Physical Chemistry (Lyons) .
PDF Representing a Reaction with a Potential Energy Diagram Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
Potential Energy Diagrams - Chemistry - Catalyst, Endothermic ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Energy Diagrams of Reactions | Fiveable Nov 23, 2021 · Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
Reaction Coordinate Diagrams The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.





















0 Response to "42 Energy Diagram For Endothermic Reaction"
Post a Comment