43 electron dot diagram for xenon
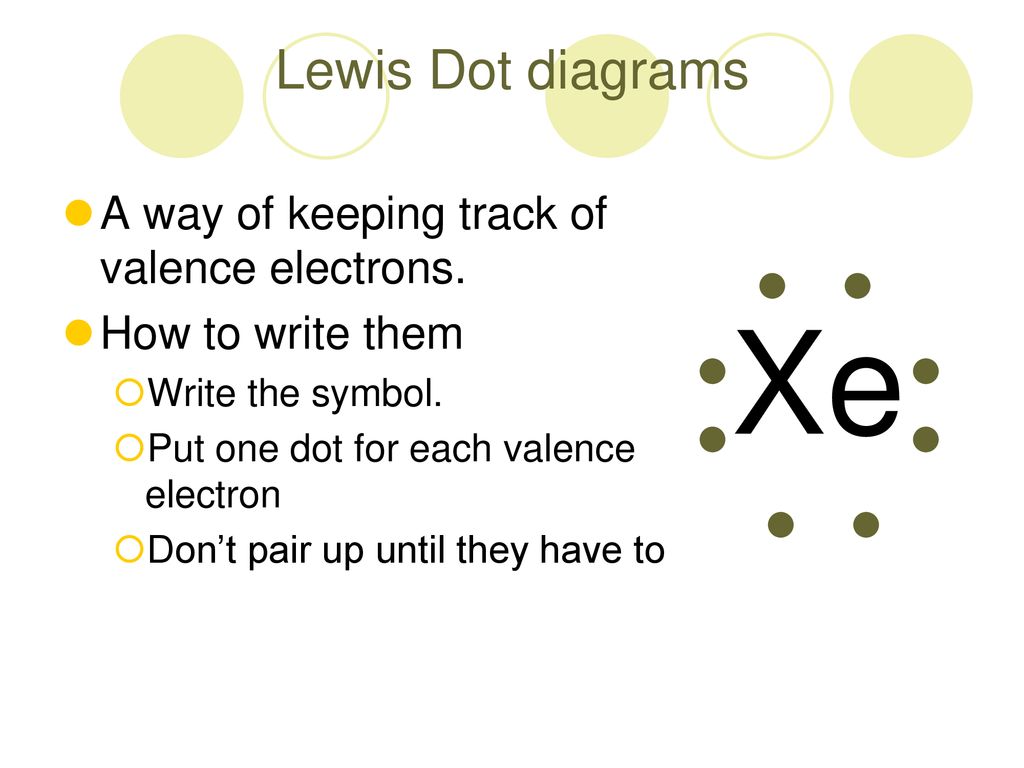
What is the electron dot diagram for xenon? - Answers The electron dot, or Lewis dot diagram for xenon is the symbol Xe surrounded by four pairs of dots, representing eight valence electrons. Refer to the related link for an illustration. What is the electron dot diagram for Helium? - Qaalot What is the electron dot diagram for Helium? Due to this fact Helium solely had 2 valence electrons. It is positioned in Group 8A as a result of it is outer shell is full with two electrons. While you draw the Lewis construction for Helium you may put two "dots" or valance electrons round the aspect image (He). Click on to see full reply.
XeO4 lewis structure, Molecular geometry, Polar or ... For xenon atom ⇒ Valence electrons of xenon = 8 ⇒ Lone pair electrons on xenon = 0 ⇒ Bonding electrons around xenon (4 single bonds) = 8 ∴ (8 - 0 - 8/2) = +4 formal charge on the xenon central atom. The above XeO4 lewis structure is not stable because of the high formal charge.
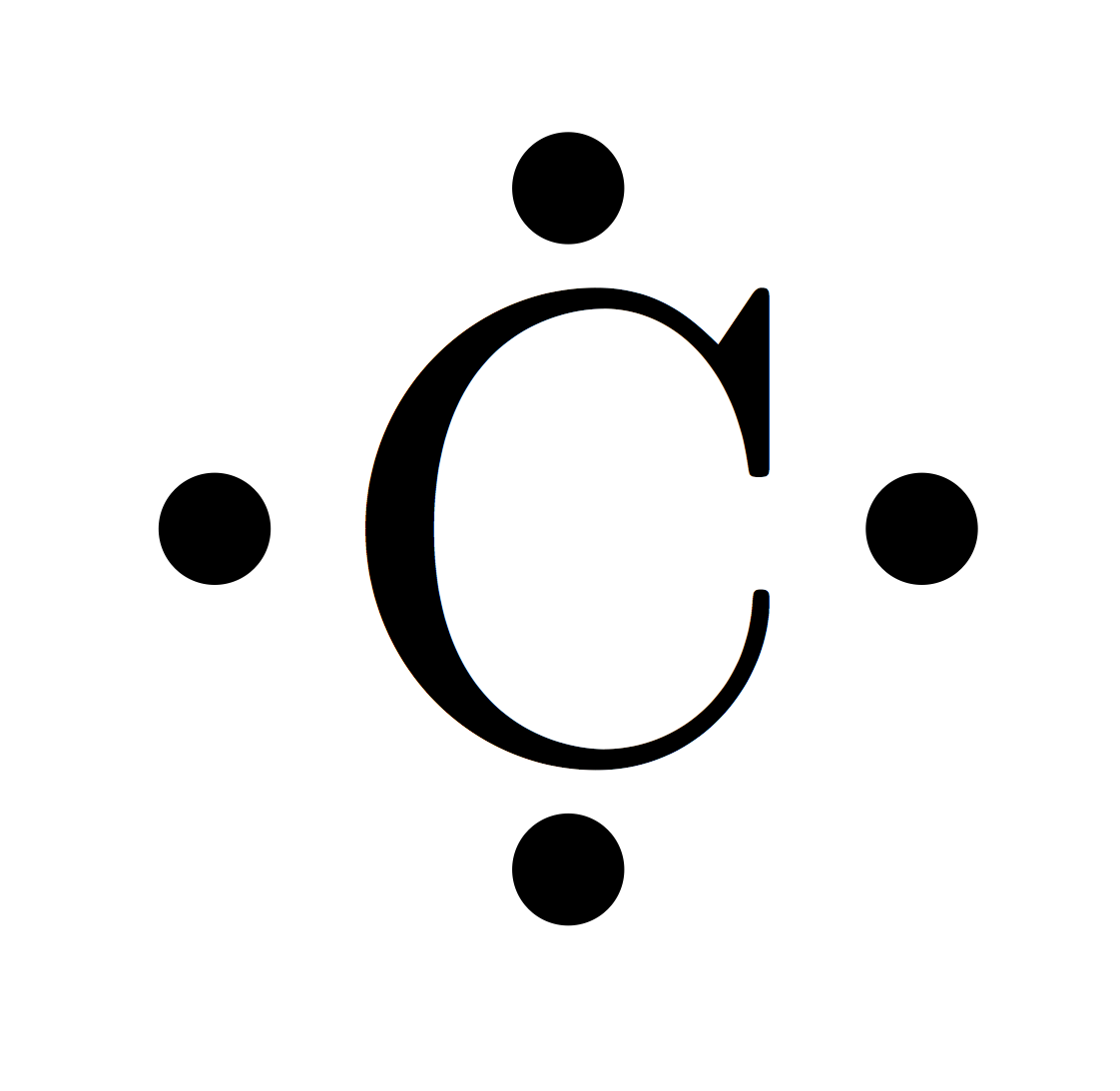
Electron dot diagram for xenon
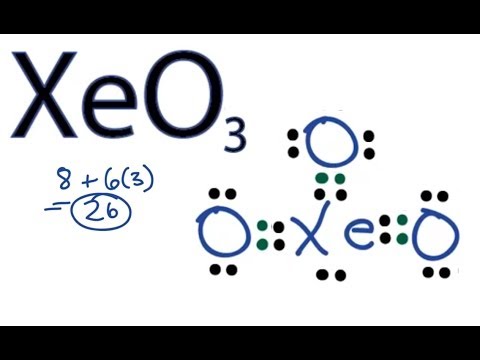
How to Draw a Lewis Structure for XeO3 xenon trioxide ... How to Draw a Lewis Structure for XeO3 xenon trioxide?Lewis Structure: ... Fluorescence - Wikipedia Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation.It is a form of luminescence.In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet … Atomic Theory – Introductory Chemistry – 1st Canadian Edition Lewis Electron Dot Diagrams. Electron Transfer: Ionic Bonds. Covalent Bonds. ... Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. ... a xenon atom with 54 protons and 77 neutrons; Americium-241 is an isotope used in smoke detectors.
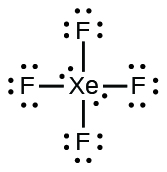
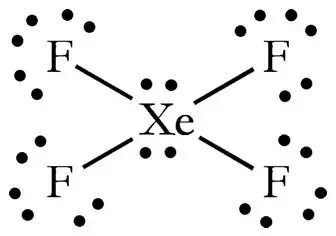
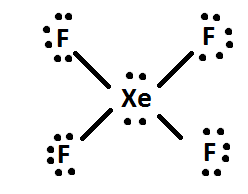
Electron dot diagram for xenon. XeF4 (Xenon tetrafluoride) Lewis Structure XeF 4 (Xenon tetrafluoride) Lewis Structure. In XeF 4 (Xenon tetrafluoride) lewis structure, there are four sigma bonds and two lone pairs around xenon atom. Each fluorine atom has three lone pairs. In this tutorial, we will learn how to draw lewis structure of XeF 4 step by step.. Lewis structure of XeF 4. Xenon atom is the center atom and each fluorine atom has made a single bond with xenon ... Lewis Dot Diagram For Xenon - schematron.org The electron dot, or Lewis dot diagram for xenon is the symbol Xe surrounded by four pairs of dots, representing eight valence electrons. Refer to the related link for an illu. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can . XeF4 Lewis Structure, Molecular Geometry, Hybridization ... There are 8 valence electrons in Xenon and 28 in that of all fluorine atoms, since there are 4 fluorine atoms in the compound. Therefore, 7*4 shall give us 28; the total valence electrons of XeF4 come to be 8+28 which is 36. Hence, there are 36 valence electrons in the compound XeF4. Xenon shows the least electronegative property. XeO4 Lewis Structure, Geometry, Hybridization, and ... Xenon is a group 18 element and therefore, has 8 valence electrons and a complete octet, Oxygen on the other hand belongs to group 16 and comprises 6 electrons in its outermost shell and therefore, needs two more electrons to satisfy its octet. Now, counting the total number of electrons for XeO4: Xenon = 8 Valence electron
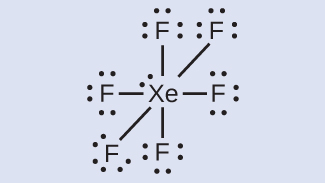
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... XeF6 Lewis Structure - How to Draw the Dot Structure for ... Drawing the Lewis Structure for XeF 6. Viewing Notes: In the XeF 6 Lewis structure Xe is the least electronegative and goes at the center of the structure.; The Lewis structure for XeF 6 requires you to place more than 8 valence electrons on Xe.; Xenon (Xe) can have more than 8 valence electrons in your Lewis structure. How to Draw the Lewis Dot Structure for Xe: Xenon - YouTube A step-by-step explanation of how to draw the Xe Lewis Dot Structure.For the Xe structure use the periodic table to find the total number of valence electron... XeF4 Lewis Structure - How to Draw the Dot Structure for ... In the XeF 4 Lewis structure Xe is the least electronegative and goes at the center of the structure. The Lewis structure for XeF 4 requires you to place more than 8 valence electrons on Xe. Xenon (Xe) can have more than 8 valence electrons in your Lewis structure. Hydrogen (H) only needs two valence electrons to have a full outer shell.
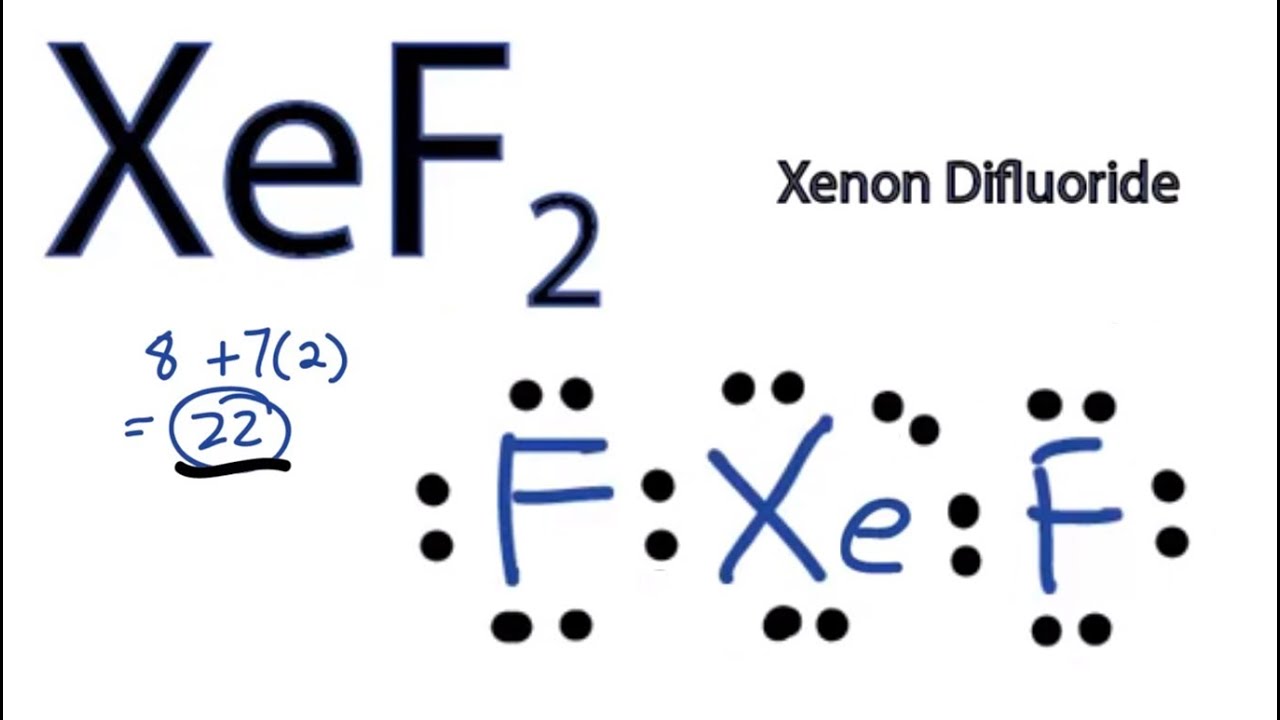
XeF2 Lewis Structure - How to Draw the Lewis ... - YouTube A step-by-step explanation of how to draw the XeF2 Lewis Dot Structure (Xenon difluroide).For the XeF2 structure use the periodic table to find the total num... XeO2F2 Lewis Structure, Geometry, Hybridization, and ... The Lewis structure of XeO2F2 is: Looking at the Lewis structure of XeO2F2, it can be deciphered that all the atoms have attained their octet. Xenon, being a noble gas, already had eight valence electrons. Also, both fluorine and oxygen atoms that were lacking one and two electrons, respectively, have become stable by attaining octet. Lewis Structure for XeF2 - UMD There are a total of 22 valence electrons in the Lewis structure for XeF2. The Lewis structure for XeF2 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons. If playback doesn't begin shortly, try restarting your device. XeOF4 Lewis Structure, Geometry, Hybridization, and ... In the case of the XeOF4 molecule, Xenon is a noble gas and a group 18th element has 8 valence electrons, Oxygen is a group 16 element and had 6 electrons in its outermost shell while fluorine is group 17 element having 7 valence electrons. Now calculating the total number of electrons in XeOF4 molecule: Xe = 8 valence electrons
PDF CHEMISTRY 2008 SCORING GUIDELINES - College Board (d) Xenon can react with oxygen and fluorine to form compounds such as XeO 3 and XeF 4. In the boxes provided, draw the complete Lewis electron-dot diagram for each of the molecules represented below. XeO 3 XeF 4 One point is earned for each correct Lewis electron-dot diagram. Omission of lone pairs of electrons on the O or F atoms
How to draw XeCl4 Lewis Structure? - Science Education and ... Key Points To Consider When Drawing The XeCl4 Structure. A three-step approach for drawing the XeCl4 Lewis structure can be used. The first step is to sketch the Lewis structure of the XeCl4 molecule, to add valence electrons around the xenon atom; the second step is to add valence electrons to the four chlorine atoms, and the final step is to combine the step1 and step2 to get the XeCl4 Lewis ...
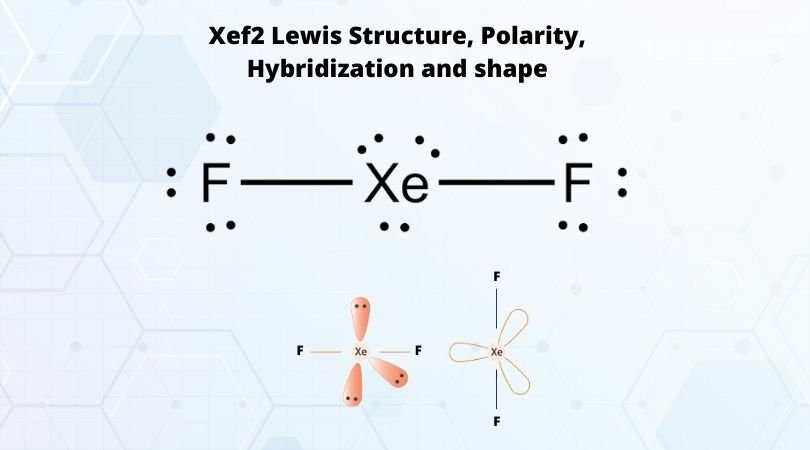
Xef2 Lewis Structure, Polarity, Hybridization and shape Xef2 Lewis Structure, Polarity, Hybridization and shape. XeF2 is an abbreviation for the chemical compound Xenon Difluoride. It is a powerful fluorinating as well as an oxidizing agent. Apart from XeF2, there are other Xenon compounds such as XeF4 ( Xenon Tetrafluoride) and XeF6 ( Xenon Hexafluoride). Out of these compounds, XeF2 is the most ...
Lewis diagram of xenon difluoride (worked example) (video ... Xenon right over here. It is a noble gas. It has eight valence electrons. One, two, three, four, five, six, seven, eight in that fifth shell. It's in the fifth period. So it has eight valence electrons. And then fluorine, we have looked at fluorine multiple times, we know that it has seven valence electrons.
How to Draw the Lewis Dot Structure for XeBr4: Xenon ... A step-by-step explanation of how to draw the XeBr4 Lewis Dot Structure.For the XeBr4 structure use the periodic table to find the total number of valence el...
Lewis Dot Diagram For Xenon - wiringall.com Xenon has 8 dots (4 pairs of dots) around the letters Xe. for XeF4. Step-by-step tutorial for drawing the Lewis Structure for XeF4. for the molecule. Remember that Xenon can have more than 8 valence electrons.
Ion source - Wikipedia Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is + + + where M is the atom or molecule being ionized, is the electron, and + is the resulting ion. The electrons may be created by an arc discharge between a cathode and an anode.. An electron beam ion source (EBIS) is used in …
Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis ... It has two lone pairs of nonbonding electrons on the central atom of Xenon. The molecule has octahedral electron geometry and square planar molecular geometry. XeF4 is a nonpolar molecule and has sp3d2 hybridization. At the Geometry of Molecules, we like knowing what you think. So let us know your thoughts on this molecule in the comments below.
Solved (a) (2 points) In the space provided below, draw a ... Chemistry questions and answers. (a) (2 points) In the space provided below, draw a Lewis structure for the molecule xenon difluoride, XeF2. If there are any atoms with a nonzero formal charge, be sure to write the formal charge next to the symbol. (b) (2 points) C The electronegativity of Xe is 2.6. The electronegativity of F is 4.0.
XeCl4 Lewis Structure: How to Draw the Lewis Structure for ... A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure (Xenon Tetrachloride)For the XeCl4 Lewis structure, calculate the total number of vale...
Xenon trioxide (XeO3) lewis structure, molecular geometry ... The xenon atom belongs to group 18th in the periodic table and oxygen is situated in the 16th group, hence, the valence electron for xenon is 8 and for oxygen atom, it is 6. ⇒ Total number of the valence electrons in xenon = 8 ⇒ Total number of the valence electrons in oxygen = 6
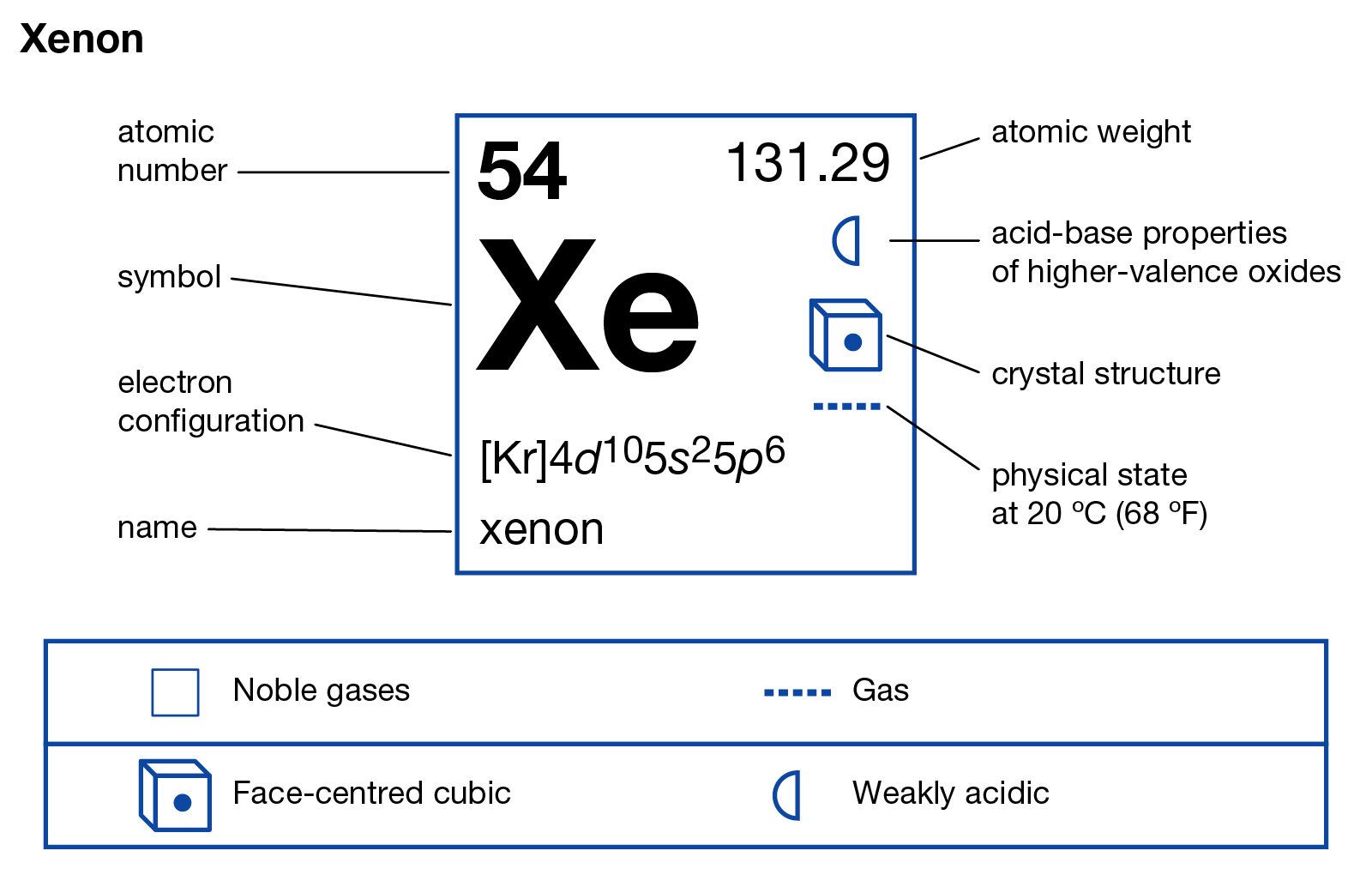
What is the electron dot notation for xenon ... So a xenon atom can give up some control of its electrons to a highly electronegative fluorine atom and form XeF₄. What information is shown in an atom's electron dot diagram? Electron dot diagrams show the valence electrons for an atom. The dot diagrams are the same for each element in the representative element groups.
Atomic Theory – Introductory Chemistry – 1st Canadian Edition Lewis Electron Dot Diagrams. Electron Transfer: Ionic Bonds. Covalent Bonds. ... Sketch a diagram of a boron atom, which has five protons and six neutrons in its nucleus. ... a xenon atom with 54 protons and 77 neutrons; Americium-241 is an isotope used in smoke detectors.
Fluorescence - Wikipedia Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation.It is a form of luminescence.In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet …
How to Draw a Lewis Structure for XeO3 xenon trioxide ... How to Draw a Lewis Structure for XeO3 xenon trioxide?Lewis Structure: ...
























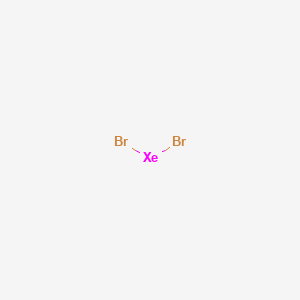
0 Response to "43 electron dot diagram for xenon"
Post a Comment