43 exothermic reaction energy diagram
Exothermic Energy Diagram: Activation Energy, Transition ... In this video, I go over how to properly label and explain a reaction mechanism diagram which is also referred to as an energy diagram or energy graph. I'll ... PDF 12.5 Exothermic & Endothermic Reactions Energy Level Diagrams The energy changes that occur during a chemical reaction can be represented by energy level diagrams. Exothermic reactions give out energy to their surroundings, and the reaction vessel may feel warm or hot when touched. Examples of exothermic reactions are: ! Combustion reactions ! Neutralisation reactions !
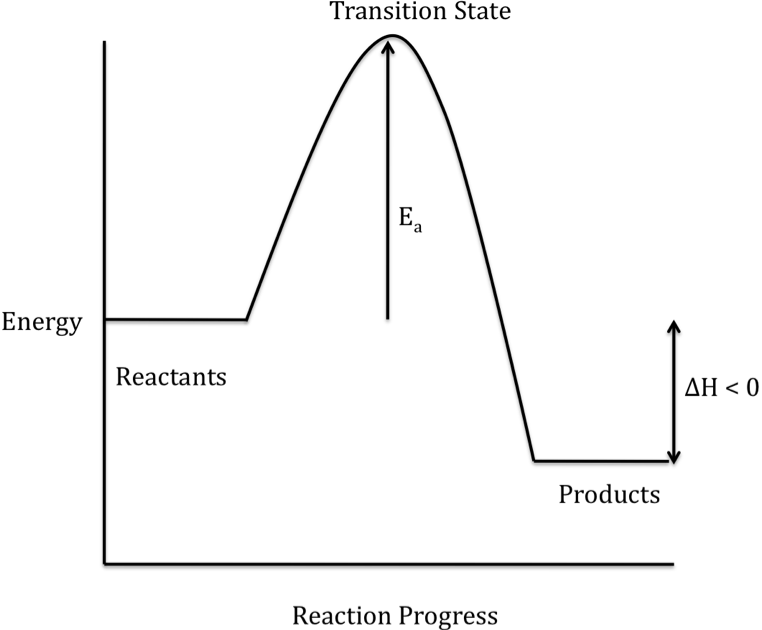
Energy Diagrams | OpenOChem Learn Energy Diagrams. Exothermic versus Endothermic Reactions. Exothermic Reactions Reactions that release heat are termed exothermic. In a exothermic reaction the resulting products have more or more stable bonds than the reactants. The ΔH of reaction for an exothermic reaction is less than zero (ΔH rxn < 0).

Exothermic reaction energy diagram
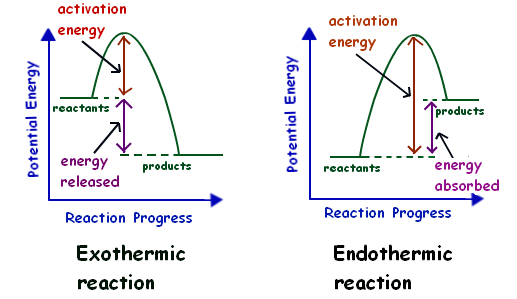
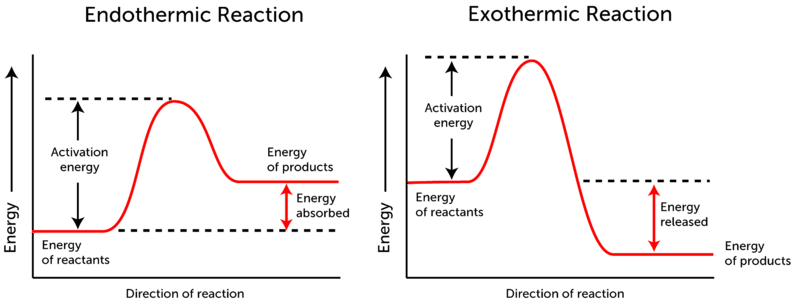
Reaction Coordinate Diagram Endothermic Vs Exothermic A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction! 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic. Representing endothermic and exothermic processes using ... Representing endothermic and exothermic processes using energy diagrams. AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state. Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... Label your diagram - if you are filling in electronically, click on diagram, click "edit" and fill in correct words in text boxes already provided. Key things to Note about EXOTHERMIC Energy Diagram: Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction (this is why products have less energy) Δ H will be negative Task 3 ...
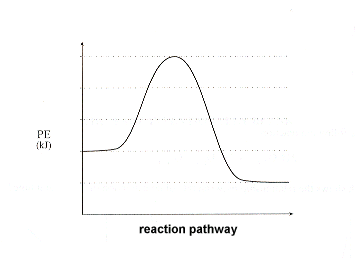
Exothermic reaction energy diagram. Energy Diagrams of Reactions | Fiveable In the graph for an exothermic reaction, you can see that the products have a lower potential energy, implying that energy has been released from the system. This further proves that ΔH is negative in an exothermic reaction. Image Courtesy of SilaVula Endothermic Reactions 5.1.2Endothermic vs Exothermic and Energy Diagrams.pdf ... Label your diagram - if you are filling in electronically, click on diagram, click "edit" and fill in correct words in text boxes already provided. Key things to Note about EXOTHERMIC Energy Diagram: Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction (this is why products have less energy) Δ H will be negative Task 3 ... Endothermic vs. exothermic reactions (article) | Khan Academy Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof. Endothermic vs. exothermic reactions. Creative Diagram Of Exothermic Reaction - Glaucoma Template You can start with a generic potential energy diagram for an exothermic reaction. A The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. There is a greater difference in energy between the reactants and products. Because the reactants have more energy than the products they are less stable.
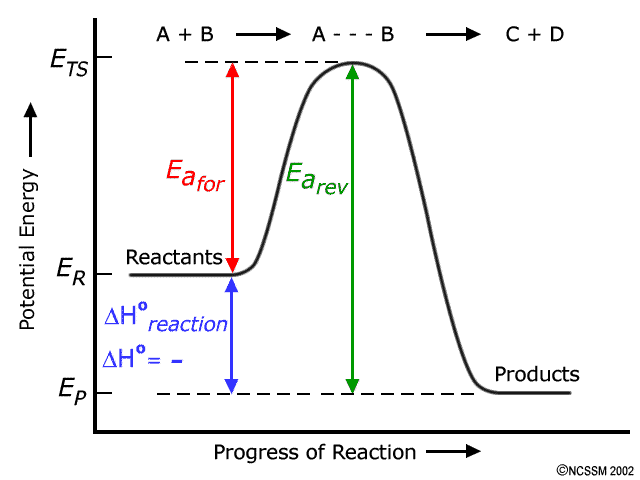
How does the energy level diagram show this reaction is ... Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. How do you know if a diagram is endothermic or exothermic ... How do you know if a diagram is endothermic or exothermic? In the energy level diagram, the enthalpies of the products are lower than that of the reactants. Hence, the enthalpy change is negative (ΔH<0). By examining this enthalpy change, one can tell whether a reaction is endothermic (ΔH>0) or exothermic (ΔH<0). PDF 5.1 - Exothermic and Endothermic Reactions 5.1 - Exothermic and Endothermic Reactions 5.1.1 - Define the terms exothermic reaction, endothermic reaction and standard enthalpy change of reaction Exothermic Reaction - A reaction that causes the temperature of the surroundings to increase. Energy is lost, or released, in the reaction, as the enthalpy of the products is less Reaction profiles - Exothermic and endothermic reactions ... An energy level diagram shows whether a reaction is exothermic or endothermic. It shows the energy in the reactants and products, and the difference in energy between them. Exothermic reaction The...
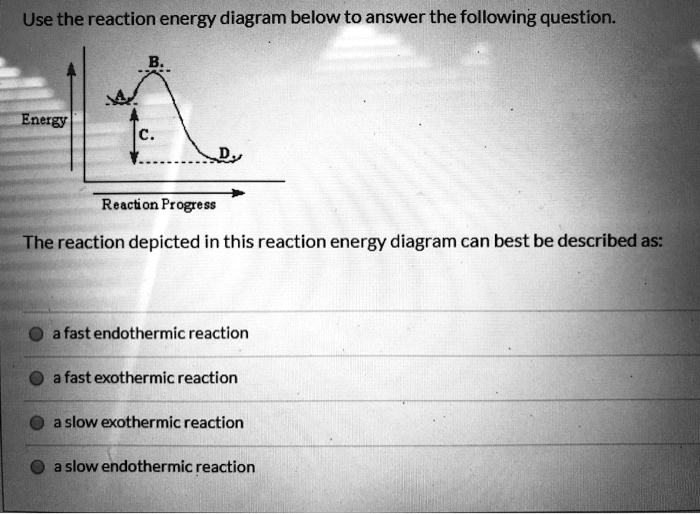
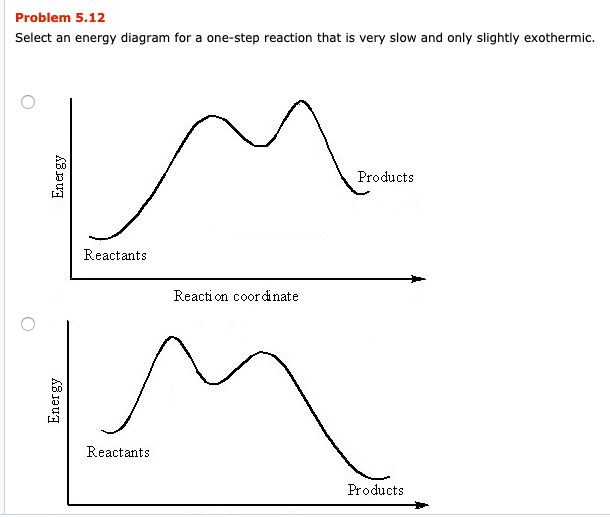
Exothermic Reaction - Definition and Examples | Properties ... Exothermic Reactions is the flow of the net transfer of heat energy during the reaction is from the medium into its surroundings. In exothermic reactions, the reactants always possess more energy than the products and hence are less stable. For this reason, the exothermic reactions require very less amount of activation energy to initiate the reaction. Exothermic And Endothermic Reactions Gcse Worksheet - Jojo ... Complete the energy profile diagram and state whether the reaction is endothermic or exothermic explain your answer. When a chemical reaction occurs energy is transferred to or from the surroundings. Topic Introducing exothermic and endothermic reactions Level Key Stage 3 or any course for students aged 11-14 Outcomes 1. Solved Based on the following energy diagram, is the ... Transcribed image text: Based on the following energy diagram, is the reaction exothermic or endothermic or neither? free energy, kJ/mol reaction coordinate O Exothermic Endothermic O Neither Which of the following is an energy diagram for a three-step reaction? free energy. kJ/mol reaction coordinate A. reaction coordinate B. reaction coordinate C. reaction coordinate D. ОА OB ОС OD Which ... Exothermic and Endothermic Processes | Introduction to ... Exothermic reaction In an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings. Endothermic Reactions. Endothermic reactions are reactions that require external energy, usually in the form of heat, for the ...
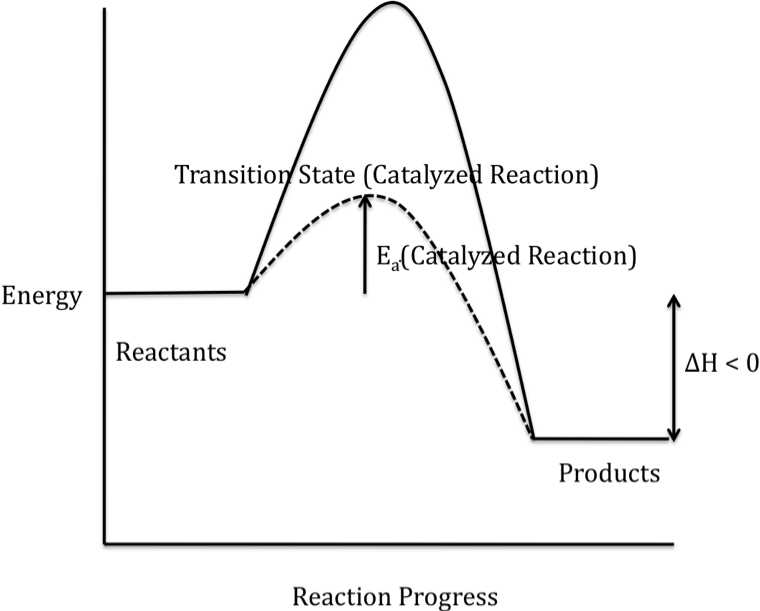
Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f...
Energy level diagrams - Why are there energy changes in ... Energy level diagrams are used to model energy changes during reactions. They show the relative energy levels of the products and reactants. Exothermic reaction The energy level decreases in an...
PPTX Energy and Chemical Reactions Exothermic Diagram. Energy released in bond making. Activation Energy. Energy used in bond. breaking. Exothermic - More energy is released when the products where formed than energy was used to break bonds in the reactants. Therefore, a net release of energy.
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ...
What are Exothermic Reactions? (with Examples and Video) An exothermic reaction is a reaction in which energy is released in the form of light or heat. Thus in an exothermic reaction, energy is transferred into the surroundings rather than taking energy from the surroundings as in an endothermic reaction. In an exothermic reaction, change in enthalpy ( ΔH) will be negative.
PPTX Energy and Reactions Energy level diagram for an exothermic chemical reaction without showing the activation energy. It could also be seen as quite exothermic with a highly unlikely zero activation energy, but reactions between two ions of opposite charge usually has a very low activation energy.
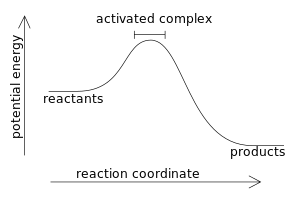
Reaction Coordinate Diagrams The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. This is an exothermic reaction(heat is given off) and should be favorable from an energy standpoint. The energy difference between A and B is E in the diagram.
Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... EXOTHERMIC Label your diagram - if you are filling in electronically, click on diagram, click "edit" and fill in correct words in text boxes already provided. Key things to Note about EXOTHERMIC Energy Diagram: Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction (this is why products have less energy) Δ H will be negative ...
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... diagram. The vertical axis on this diagram represents the energy level and the horizontal axis represents the progress of the reaction from reactants to products. Energy level diagrams for exothermic reactions In an exothermic reaction, reactants have more energy than the products . The difference between
Endothermic and Exothermic Reactions Diagram | Quizlet Diagram of endothermic and exothermic reactions. Terms in this set (5) Exothermic Reaction In this type of reaction, energy (in the form of heat, sound or light) is released when the reactants break apart. Heat energy can be picked up by the area surrounding the products. This means that there was more energy in reactants than in the products.
How does the energy level diagram show this reaction is ... Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases.
Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... Label your diagram - if you are filling in electronically, click on diagram, click "edit" and fill in correct words in text boxes already provided. Key things to Note about EXOTHERMIC Energy Diagram: Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction (this is why products have less energy) Δ H will be negative Task 3 ...
Representing endothermic and exothermic processes using ... Representing endothermic and exothermic processes using energy diagrams. AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
Reaction Coordinate Diagram Endothermic Vs Exothermic A Reaction Coordinate (Energy) Diagram Thermodynamic Quantities Gibbs standard free energy change there are many ENDOTHERMIC reactions such as photosynthesis that occur.! An Exothermic (Exergonic) Reaction! 2 C 8 H 18 + 25 O 2 → 16 CO 2 + 18 H 2 O! A reaction with ∆H°0 is endothermic.














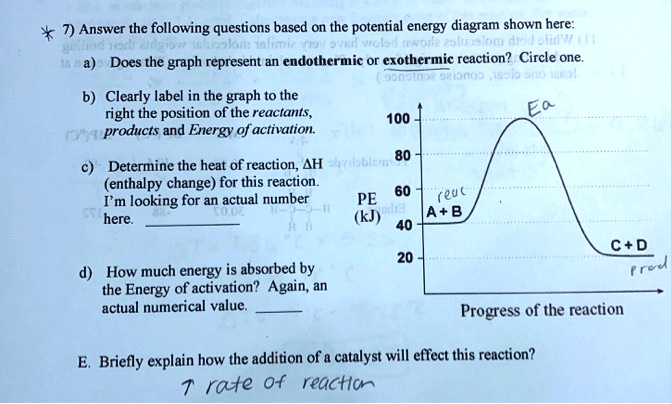








0 Response to "43 exothermic reaction energy diagram"
Post a Comment