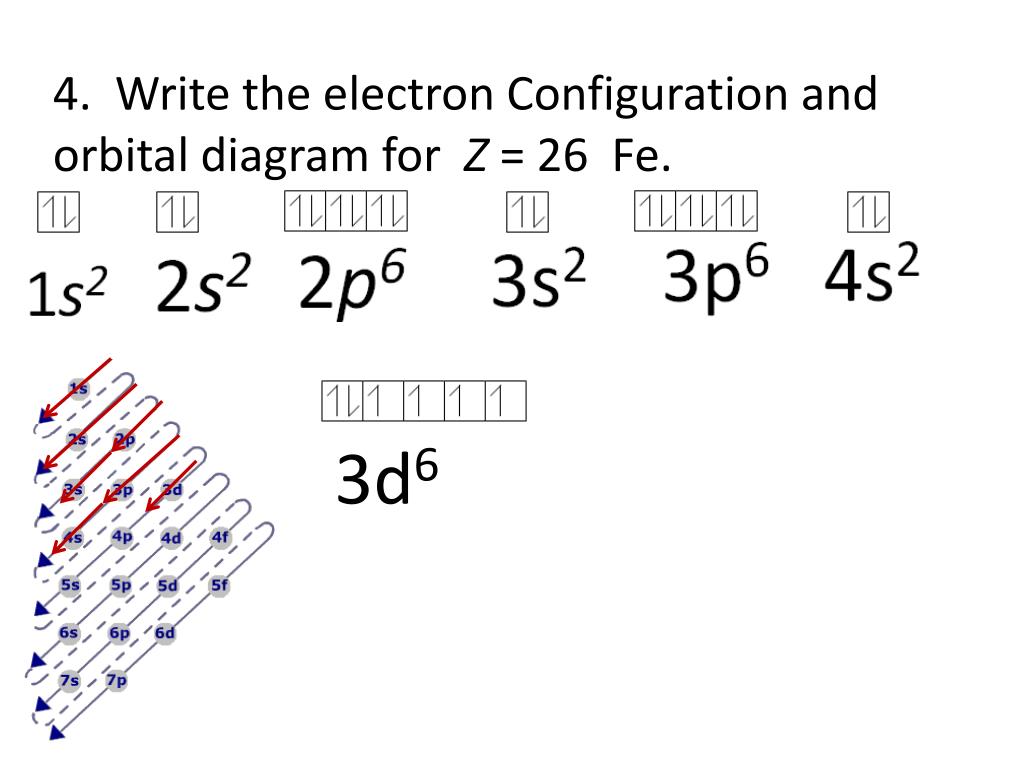
44 orbital diagram for fe
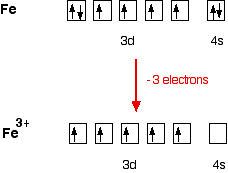
42 fe2+ orbital diagram - Wiring Diagrams Manual Sketch an atomic orbital diagram for Fe2 in its ground state. A normal Fe atom has an electron arrangement of. Paramagnetic With One Unpaired Electronb. The Ground State Electron Configuration Of Fe2 Ion Is 1s2 2s2 2p6 3s2 3p6 3d6 Therefore Fe2 Is. Molecular orbital (SCF-X-α-SW) theory of Fe 2+- Mn 3+ , Fe ... Bonding in Metal Carbonyls: Explaination, Type, Property ... To understand the bonding in metal carbonyls, we need to first learn the Molecular Orbital \ (\left ( { {\rm {MO}}} \right)\) diagram of carbon monoxide. There are ten electrons in the carbon monoxide ligand. The order of energy of the molecular orbitals and the accommodation of ten electrons of the carbon monoxide can be shown as:
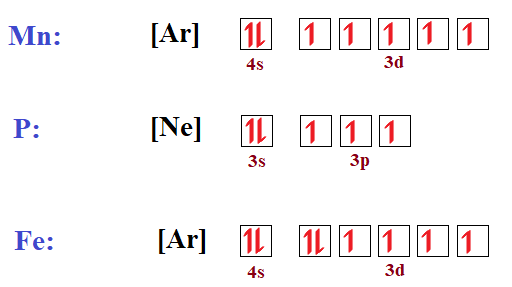
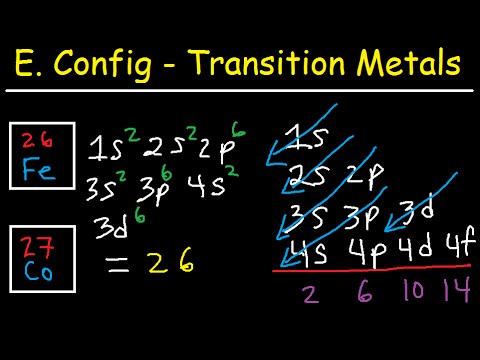
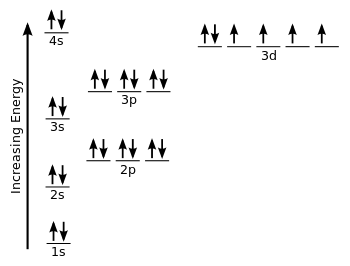
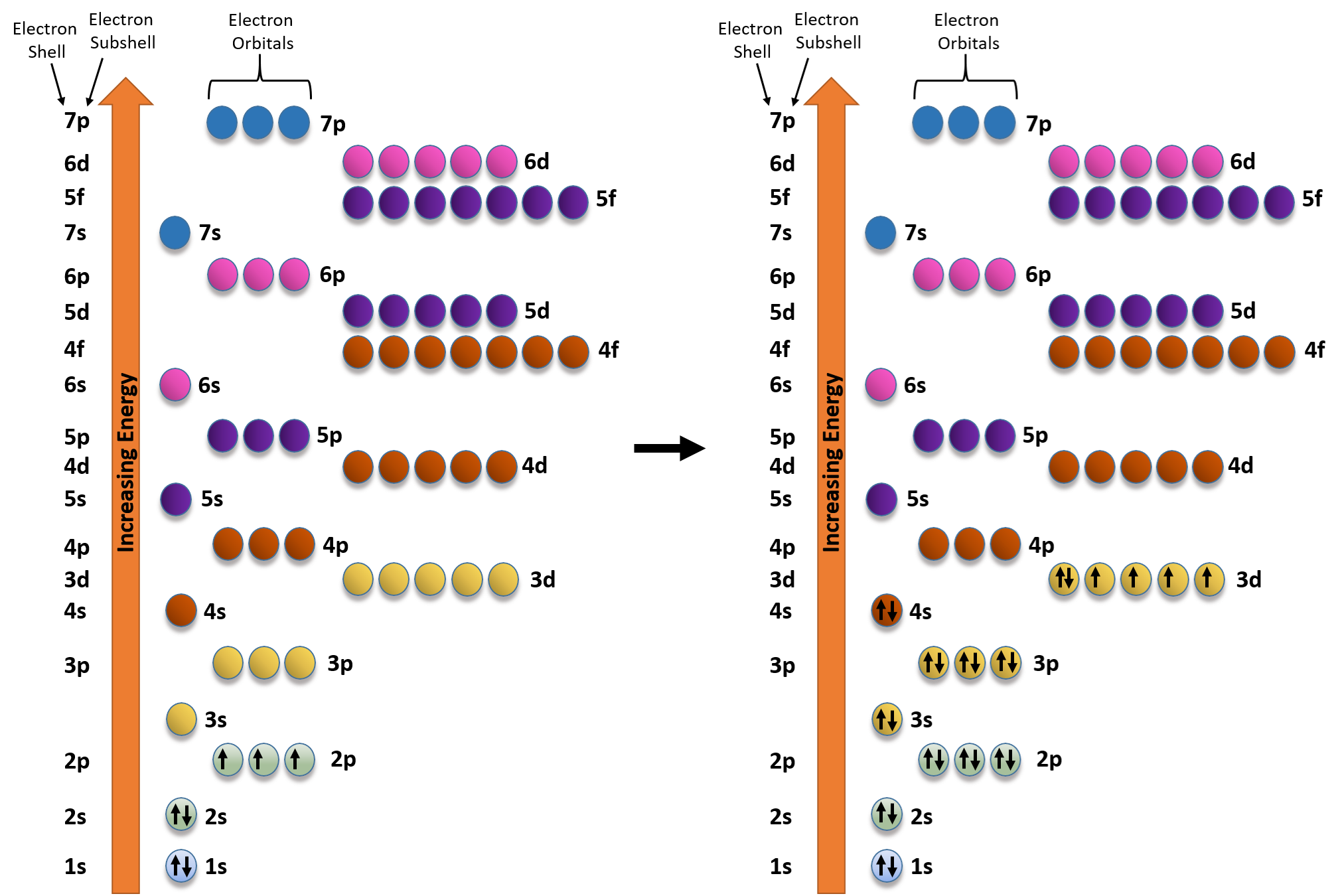
Write an orbital diagram for the ground state of iron atom ... Orbital diagram for the ground state of the Phosphorous atom (Z=15). Electronic configuration will be 1s²,2s²,2p⁶,3s²,3p³ The arrangement of electrons around the nucleus of an atom with a lower energy level is the statement as per AUFBAU's RULE. After 4s is full the remaining electron enters into the 3d orbital.
Orbital diagram for fe
Comparison of hexagonal boron nitride and MgO tunnel ... (a) Atomic model of an abrupt FeCo|MgO(100) interface. (b) Spin-up and spin-down density of states for interfacial MgO layers and Fe,Co planes, showing stabilization of perpendicular metal dz2 states and a model of PMA. (c) Molecular orbital diagram of the interactions. 39 construct the orbital diagram of the f ion - Diagram ... Iron (Fe) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. Orbital Diagram For Au+ - schematron.org Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+. 9.3: Electron Configurations- How Electrons Occupy ... The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium.
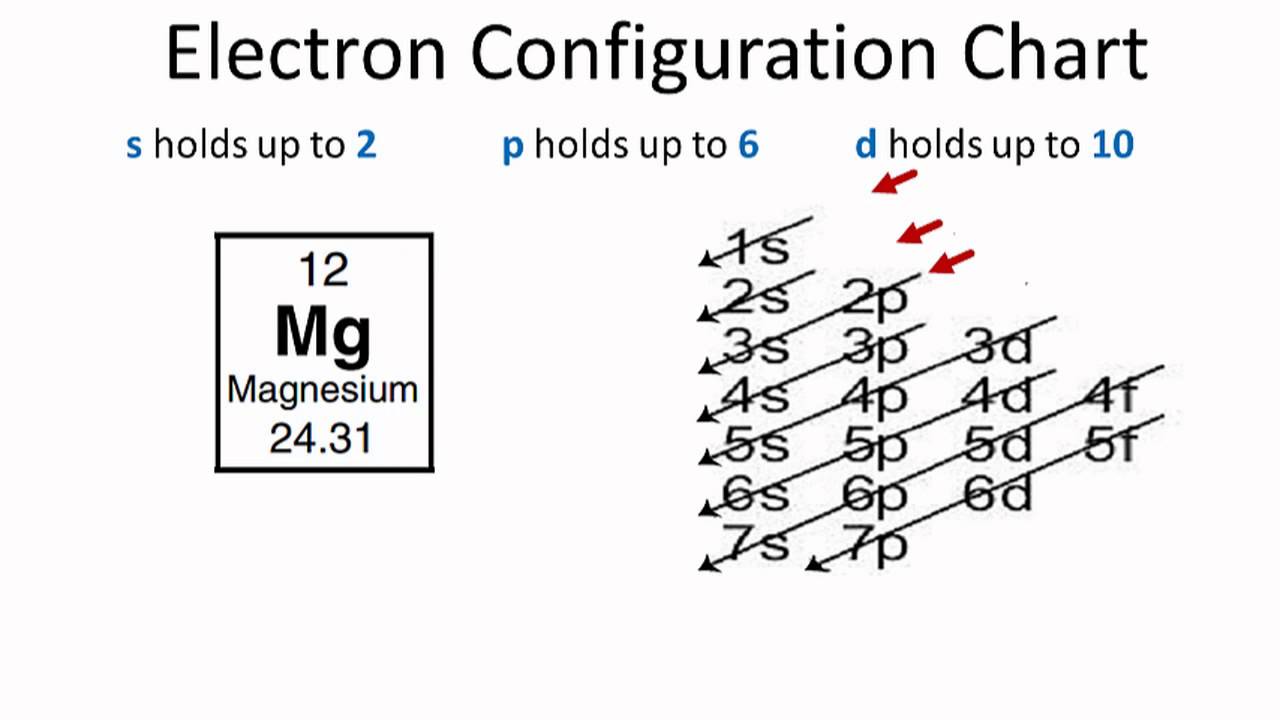
Orbital diagram for fe. Insights into the activity of single-atom Fe-N-C catalysts ... In this work, we demonstrate that the hybridization among Fe 3 dz2, 3 dyz (3 dxz ), and O 2 π* orbital is the origin of ORR activity on the Fe-N 4 site by density functional theory (DFT)... 37 orbital diagram for ge - Diagram Online Source PDF Quantum Numbers Worksheet with Orbital Diagrams CHEMISTRY 151 - ORBITAL DIAGRAMS AND ELECTRON CONFIGURATIONS KEY 1. Write a complete orbital diagram and electronic configuration for germanium, Ge. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 T T Q Q Q T T E 2 & 3. Write abbreviated electronic configurations for the following. valenceelectrons.com › iron-electron-configurationIron(Fe) electron configuration and orbital diagram Atomic Orbital Diagram for Iron (Fe) Iron ion (Fe 2+ ,Fe 3+) electron configuration Ground state electron configuration of iron (Fe) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2. The electron configuration shows that the last shell of iron has two electrons and the d-orbital has a total of six electrons. What are the electron configurations for the Ni2 and P3 ... The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s22s22p63s23p3. What element is 1s2 2s2 2p6 3s2 3p6 4s1 3d10?
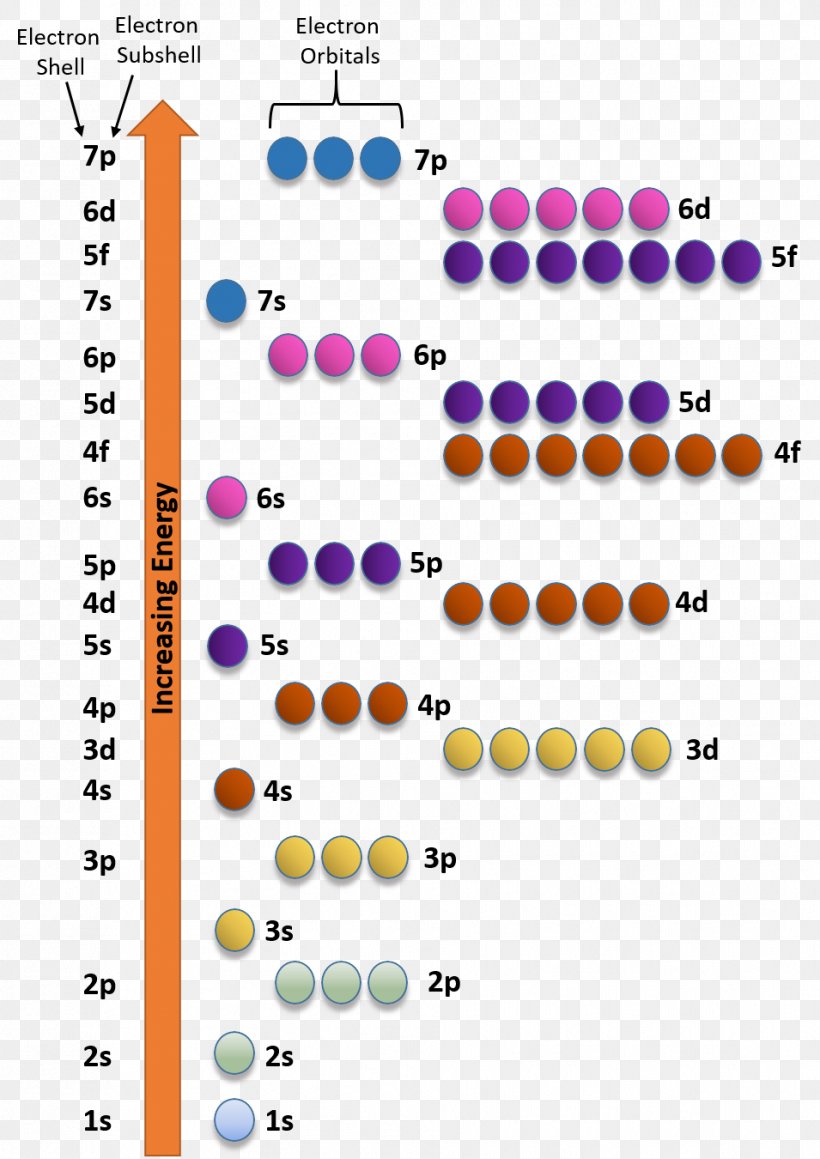
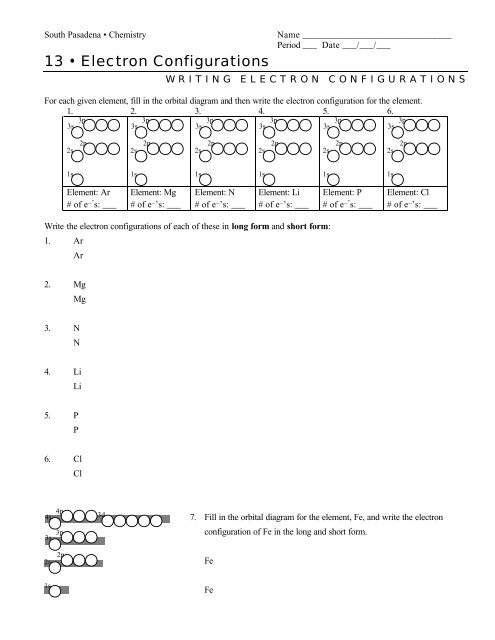
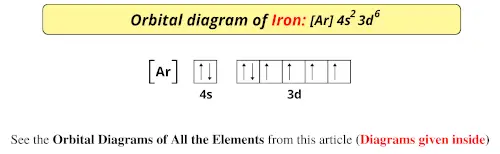
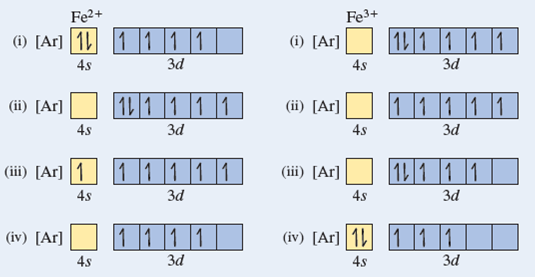
diagramweb.net › orbital-diagram-for-fe3Orbital Diagram For Fe3+ Sep 16, 2018 · Orbital Diagram For Fe3+ Atomic orbitals, electron configurations and the. Periodic Table. Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). 1) K19 Electron configuration: Orbital diagram: 2) N ... A rrows in an orbital diagram represent the electrons of a specific atom or element. There are three rules that are utilized in orbital diagrams. These are the Auf bau principle, Pauli Exclusion Principle, and Hund's Rule. According to the Auf Bau Principle, the electrons occupy the lowest energy orbital first before occupying the highest energy. What Is the Abbreviated Electron Configuration for Silver ... What is the electron configuration and orbital diagram of: Na + P 3- Al 2+ Fe 2+ Sm 3+ Solution. First, write out the electron configuration for each parent atom. We have chosen to show the full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also ... Orbital Diagrams And Electron Configuration Worksheet ... We could also say the fe electron configuration in orbital notation is [ar]4s 2 3d 6 or even [ar]3d 6 4s 2. Orbital diagrams and electron configuration worksheet answer key. Electron configuration and orbital diagram worksheet. Phosphorus 1s 2s 2p 3s 3p 4s 3d 4p 2. An electron configuration is a shorthand notation that is used to describe the ...
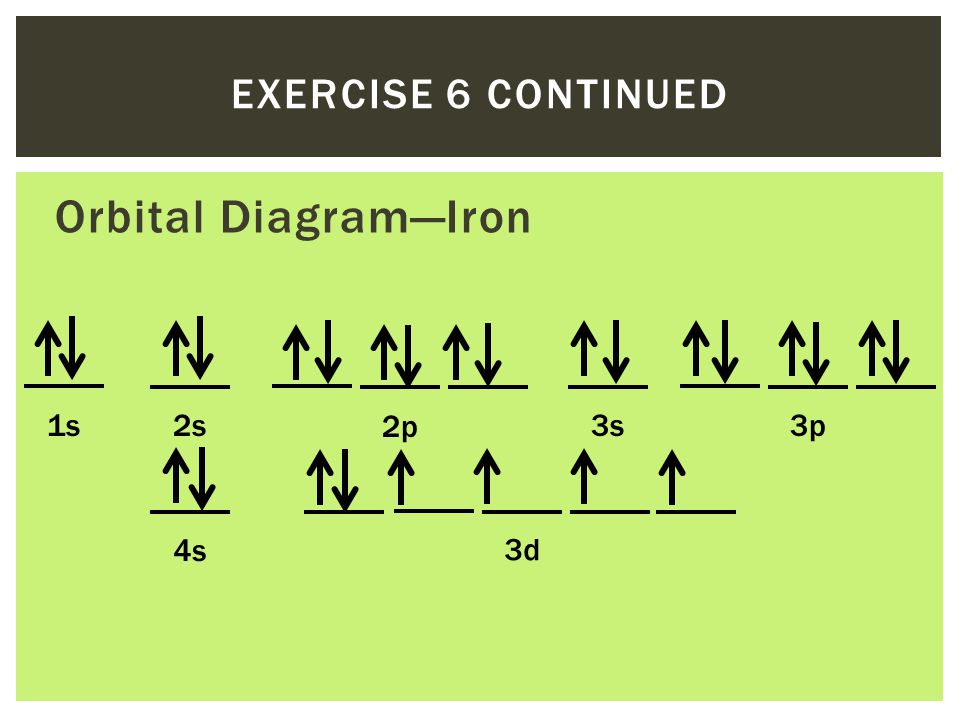
Correlation-driven electronic reconstruction in FeTe1−xSex ... Across the FeTe 1−x Se x phase diagram, we observe a large dynamic range of the relative coherence of the localized d xy orbital to that of the other itinerant Fe 3d orbitals. (Get Answer) - Write the abbreviated electron ... 10. Write the electron configuration and draw the orbital diagram for iron (Fe), atomic number 26. Use the Noble core abbreviation. Electron Configuration of neutral iron: Orbital Diagram: Write the electron configuration and draw the orbital... How Can We Find A Electron Configuration For Bromine (Br) There are 35 arrows in the Electron Configuration For Bromine, which is for orbital filling. These 35 arrows of bromine are only due to the atomic number of bromine. The orbital filling order is 1s, 2s, 3p, 3s, 4s, 3d, 4p, etc. You can see that 3p is coming before the 4p and after the 4s. Correlation between Spin and Orbital Dynamics during Laser ... The U.S. Department of Energy's Office of Scientific and Technical Information
Fe Electron Configuration - electron configuration for fe ... Fe Electron Configuration - 16 images - solved write the full electron configuration lef, electron configuration for fe3 slidedocnow, tang 02 wave quantum mechanic model, new chm 151 unit 4 power points,
› general-science › What_is_theWhat is the orbital diagram for Fe? - Answers Sep 17, 2014 · What is the orbital diagram for Fe? - Answers Fe, or iron, has the atomic number of 26. diagram is 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Home Subjects Math Science 🏛️ History Arts & Humanities Social...
la configuración electrónica y el diagrama de orbitales de ... la configuración electrónica y el diagrama de orbitales de Fe = 26 ; Ge = 32 La configuración electrónica va salir del numero atómico que tiene ese elemento , ese número ya viene planteado en el problema. La configuración electrónica del hierro (Fe) debe de ser igual a 26 electrones .
orbital diagram of k1 9 Fe 26 br 35 - Brainly.ph Orbital diagram of k1 9 - 22180722 ericbrentsalazar0 ericbrentsalazar0 18.11.2021 Science Junior High School answered Orbital diagram of k1 9 Fe 26 br 35 Na 11 P 15 1 See answer Advertisement Advertisement franzjo12yahoocom franzjo12yahoocom
schematron.org › fe2-orbital-diagramFe2+ Orbital Diagram - schematron.org Nov 09, 2018 · You're removing 2 electrons from it to generate the Fe2+ ion, which are removed from the 4s orbital first (this is always the case in transition chemistry - as far as. The electron configuration for Fe2+ will be 1s2 2s2 2p6 3s2 3p6 4s2 3d4 there is a special stabilty needed to balance the number of electrons in the 3d orbital.
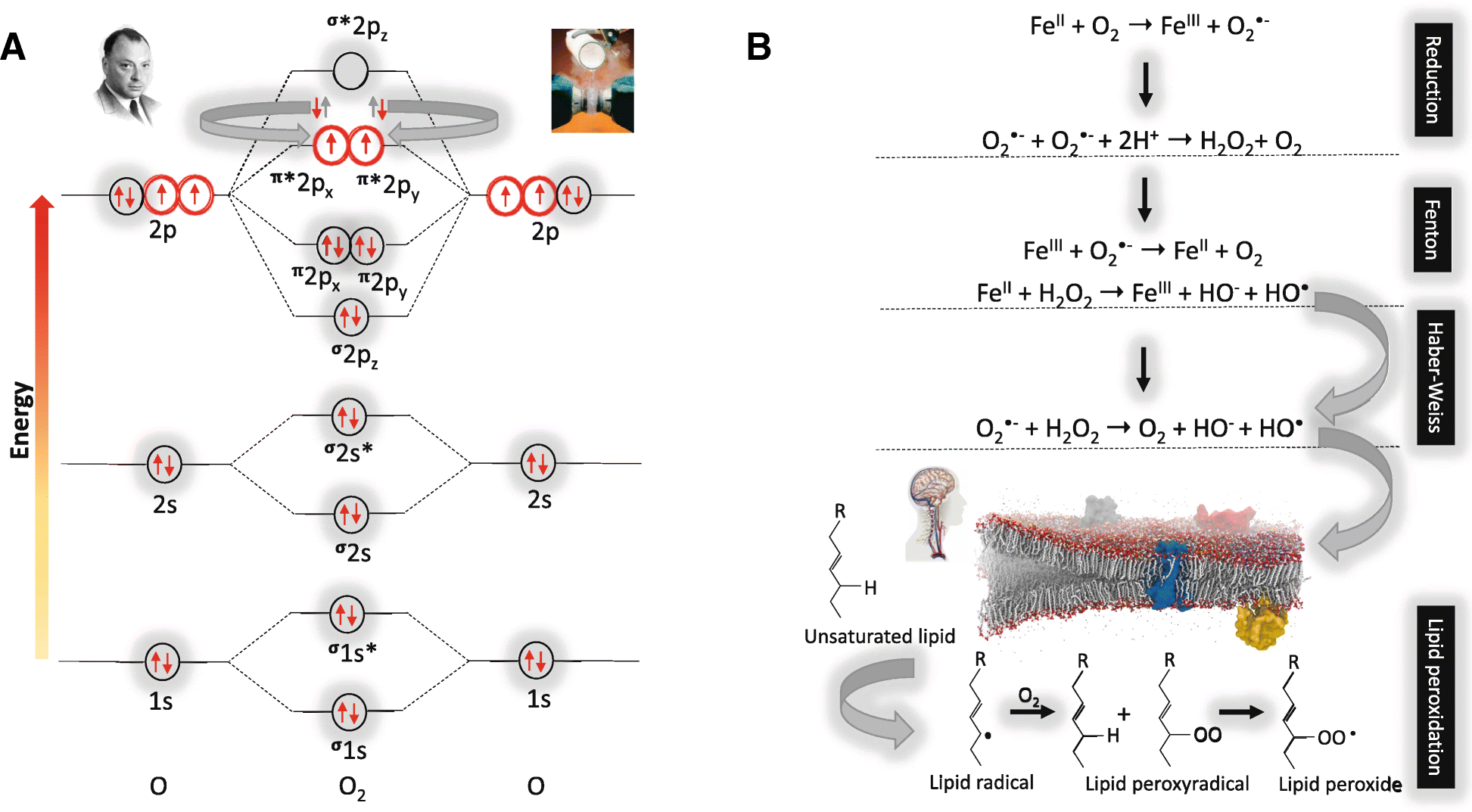
Molecular orbital study of Fe(II) and Fe(III) complexation ... The Fe Pourbaix diagram published by Takeno ( 2005) that is relevant to chemical conditions found in soils is redrawn in Figure 1. The region between the two dashed red lines is where water is redox stable. In highly oxidizing conditions, water will be oxidized to O 2, and in highly reducing conditions, it will be reduced to H 2 (Bleam, 2012 ).
Iron (Fe) - Periodic Table (Element Information & More) So the ground state of Iron is Fe. And the ground state electronic configuration of Iron is [Ar] 4s 2 3d 6. In this state, if we see the electron configuration of Iron, then it possesses incomplete d-orbitals. ... Orbital Diagram of All Elements (Diagrams given Inside)
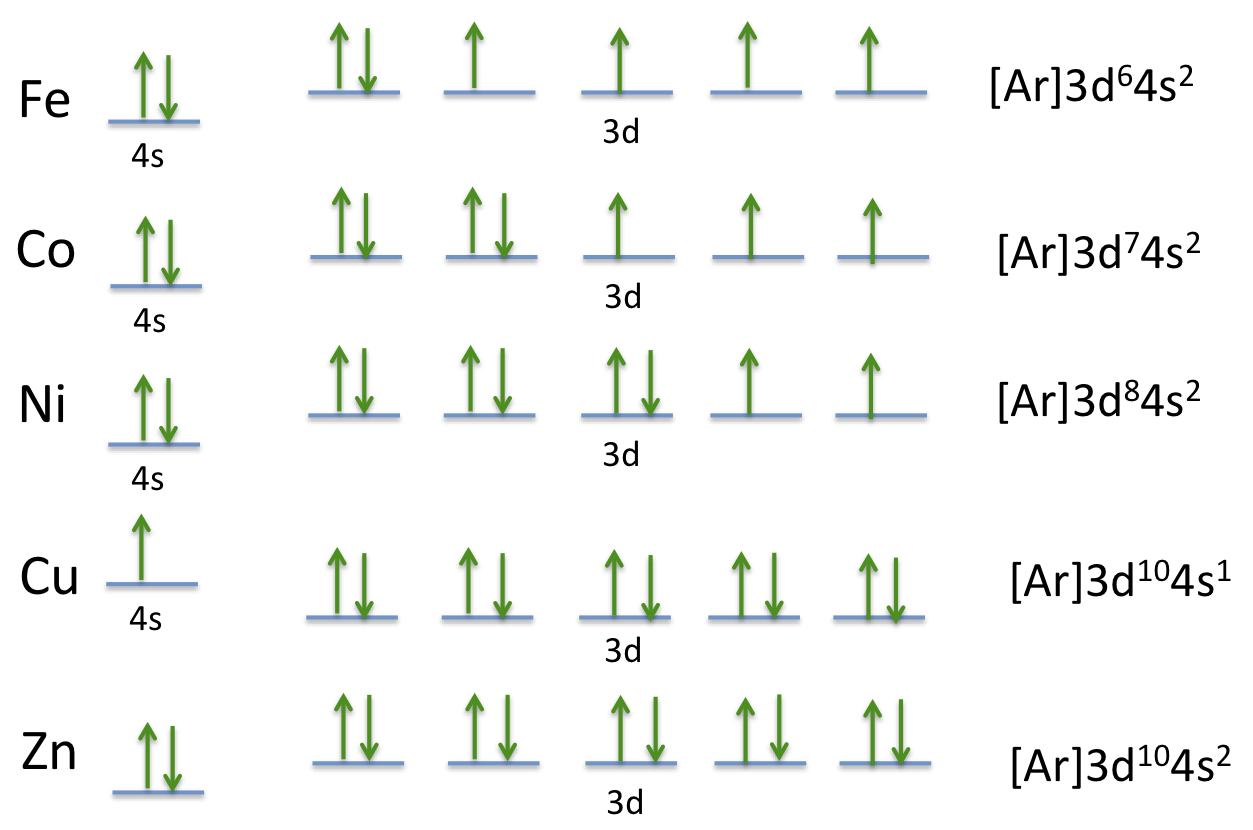
Valence Electrons Chart for All Elements (Full Chart Inside) Valence electrons in Iron (Fe) 8: 27: Valence electrons in Cobalt (Co) 9: 28: Valence electrons in Nickel (Ni) 10: 29: Valence electrons in Copper (Cu) 11: 30: Valence electrons in Zinc (Zn) 12: 31: Valence electrons in Gallium (Ga) 3: 32: Valence electrons in Germanium (Ge) 4: 33: Valence electrons in Arsenic (As) 5: 34: Valence electrons in ...
Electron Configuration Worksheet 3 Answer Key → Waltery ... Electron Configurations and Periodic Trends 1. Rt has three extra electrons 11 02 2 12. Orbital Diagrams Doc Chemistry Classroom Teaching Chemistry Electron Configuration Just fancy it by voting.Electron configuration worksheet 3 answer key. The electron configurations in this worksheet assume that lanthanum la is the first element in the 4f block and that actinium […]
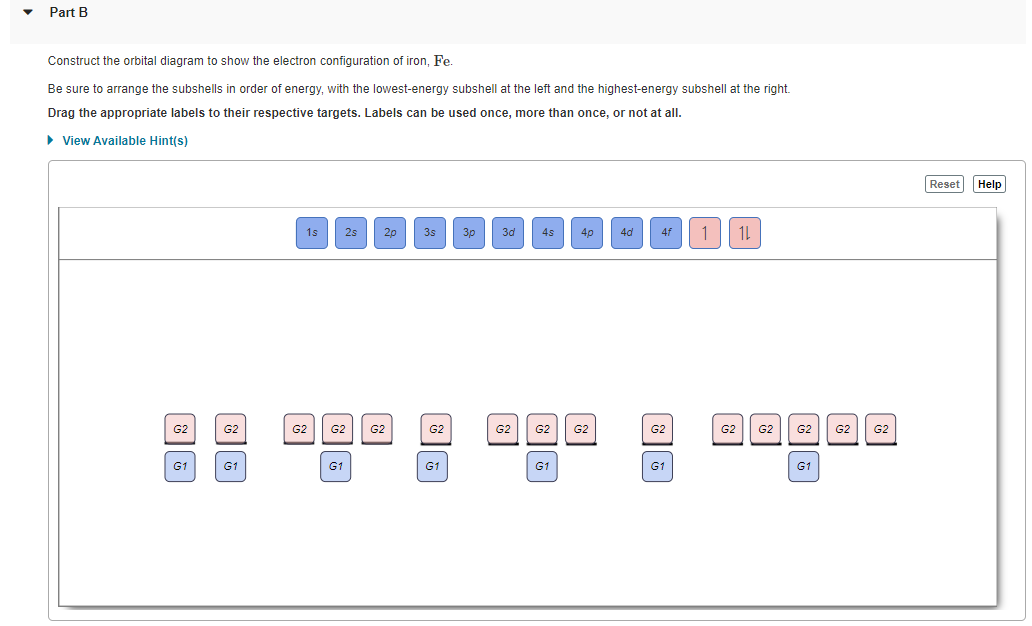
(Get Answer) - Use The Orbital-Filling Diagram To Show The ... Use The Orbital-Filling Diagram To Show The Electron Configuration Of Iron, Fe Be Sure To Arrange The Subshells In Order Of Energy, With The Lowest-Energy Subshell At The Bottom And The Highest-Energy Subshell At The Top. Use The Buttons At The Top Of The Tool To Add Sublevels. Click Within An Orbital To Add Electrons. View Available Hint (S)
Maharashtra Board Class 12 Chemistry Solutions Chapter 9 ... Draw a qualitatively energy-level diagram showing d-orbital splitting in the octahedral environment. Predict the number of unpaired electrons in the complex [Fe(CN) 6] 4⊕. Is the complex diamagnetic or paramagnetic? Is it coloured? Explain. Answer: (A) r-orbital splitting in the octahedral environment : (B) [Fe (CN) 6] 4-is an octahedral complex.
[CoCl4]^2⊕ is a tetrahedral complex. Draw its box orbital ... Draw its box orbital diagram. State which orbitals participate in hybridization. coordination compounds; class-12; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Oct 9, 2021 by PulkitKumar (35.2k points) selected Oct 10, 2021 by RakshitKumar . Best answer. Since CI ...
9.3: Electron Configurations- How Electrons Occupy ... The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium.
39 construct the orbital diagram of the f ion - Diagram ... Iron (Fe) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. Orbital Diagram For Au+ - schematron.org Figure A periodic table of partial ground- state electron configurations. Figure Write orbital diagram for Au+.
Comparison of hexagonal boron nitride and MgO tunnel ... (a) Atomic model of an abrupt FeCo|MgO(100) interface. (b) Spin-up and spin-down density of states for interfacial MgO layers and Fe,Co planes, showing stabilization of perpendicular metal dz2 states and a model of PMA. (c) Molecular orbital diagram of the interactions.



















![Molecular Orbital Theory (MOT), Bonding in [Fe(CN)6]4– ion ...](https://ebrary.net/htm/img/33/1908/304.png)

0 Response to "44 orbital diagram for fe"
Post a Comment