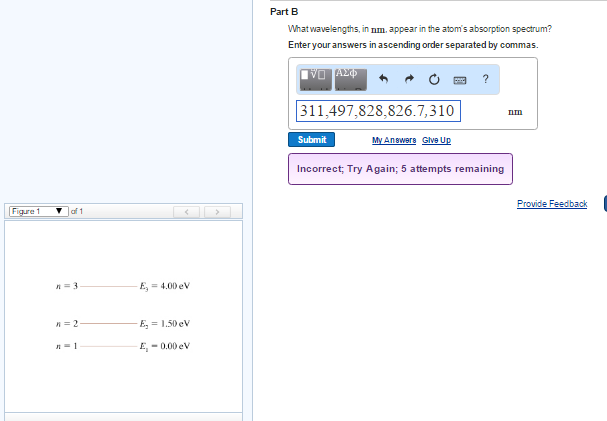
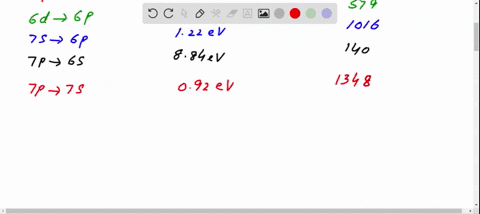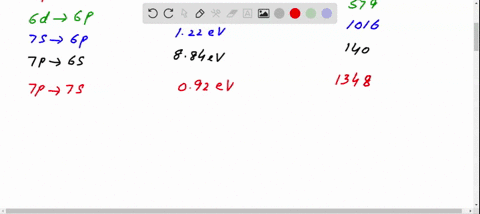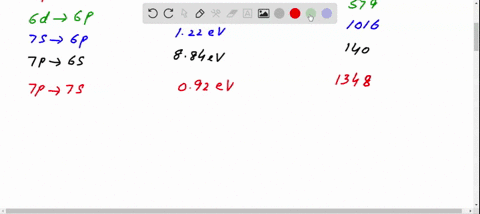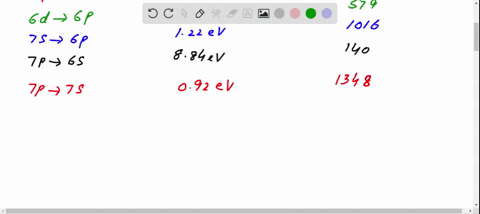
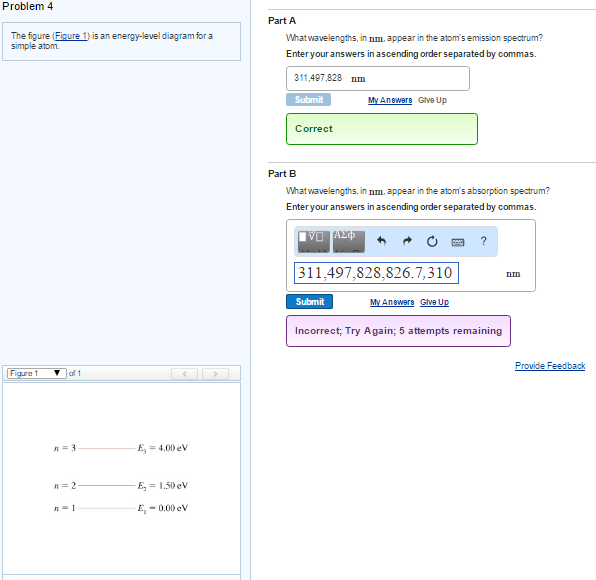
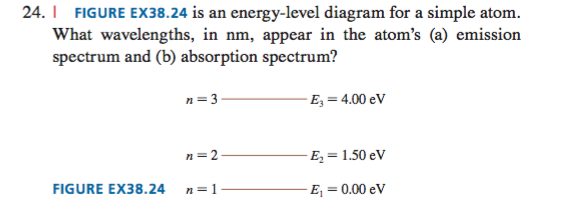
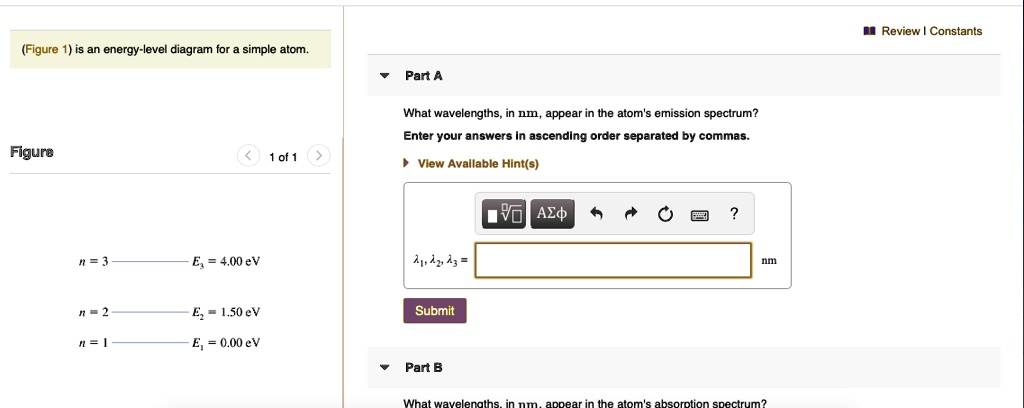
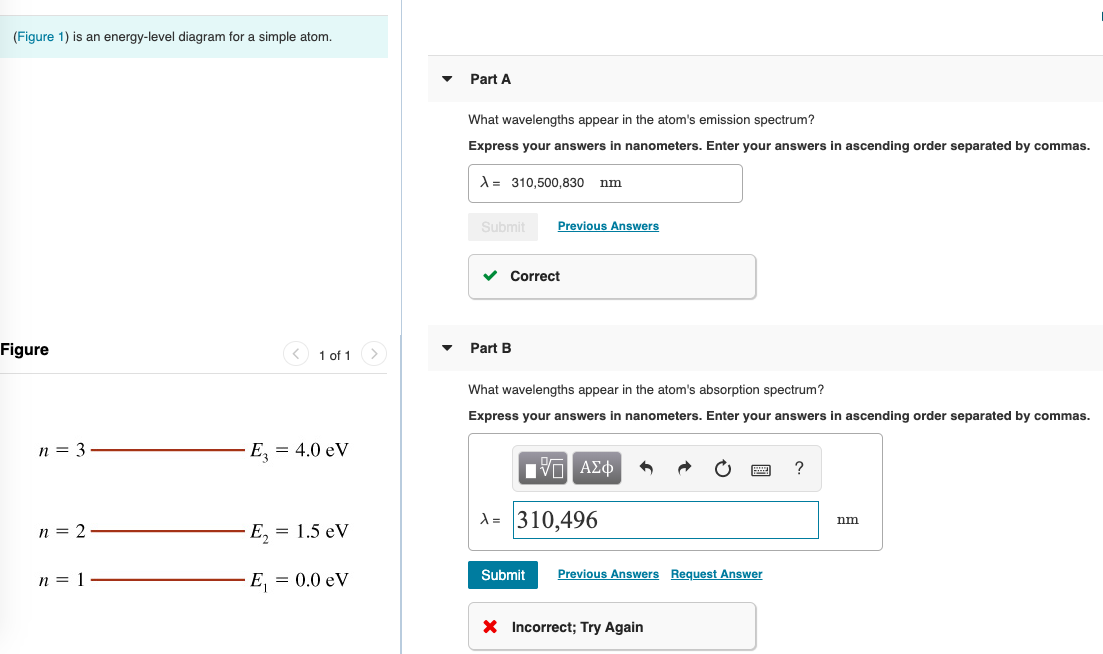
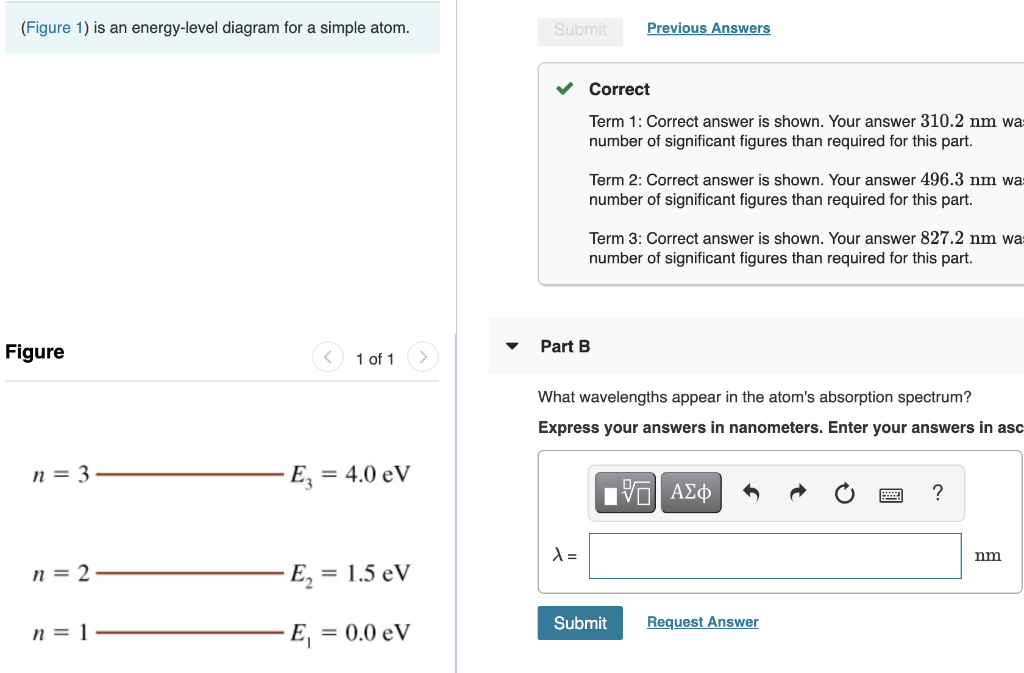
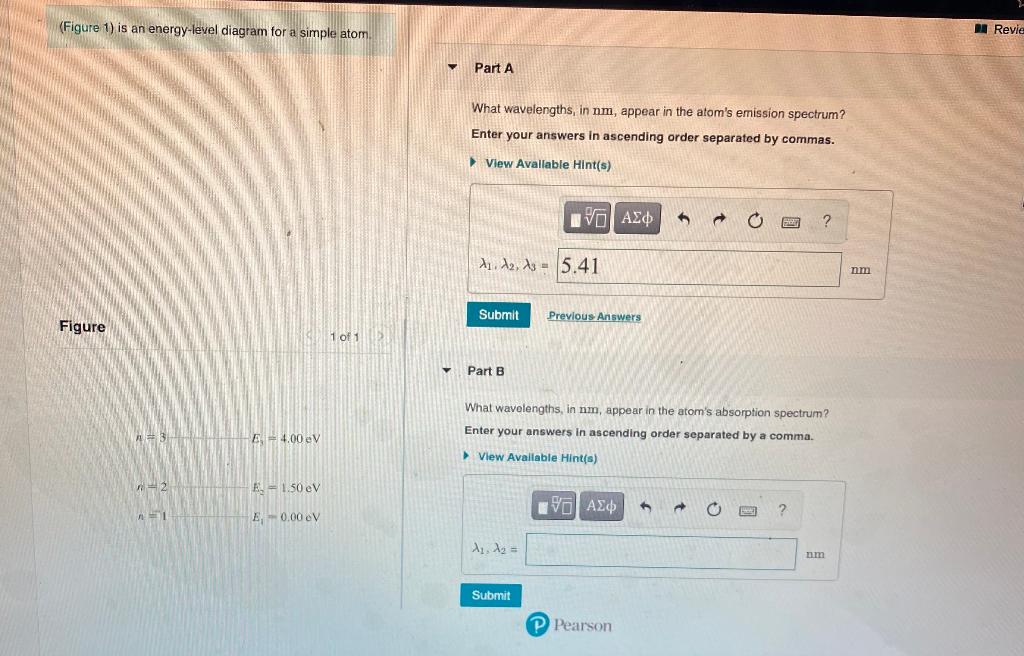
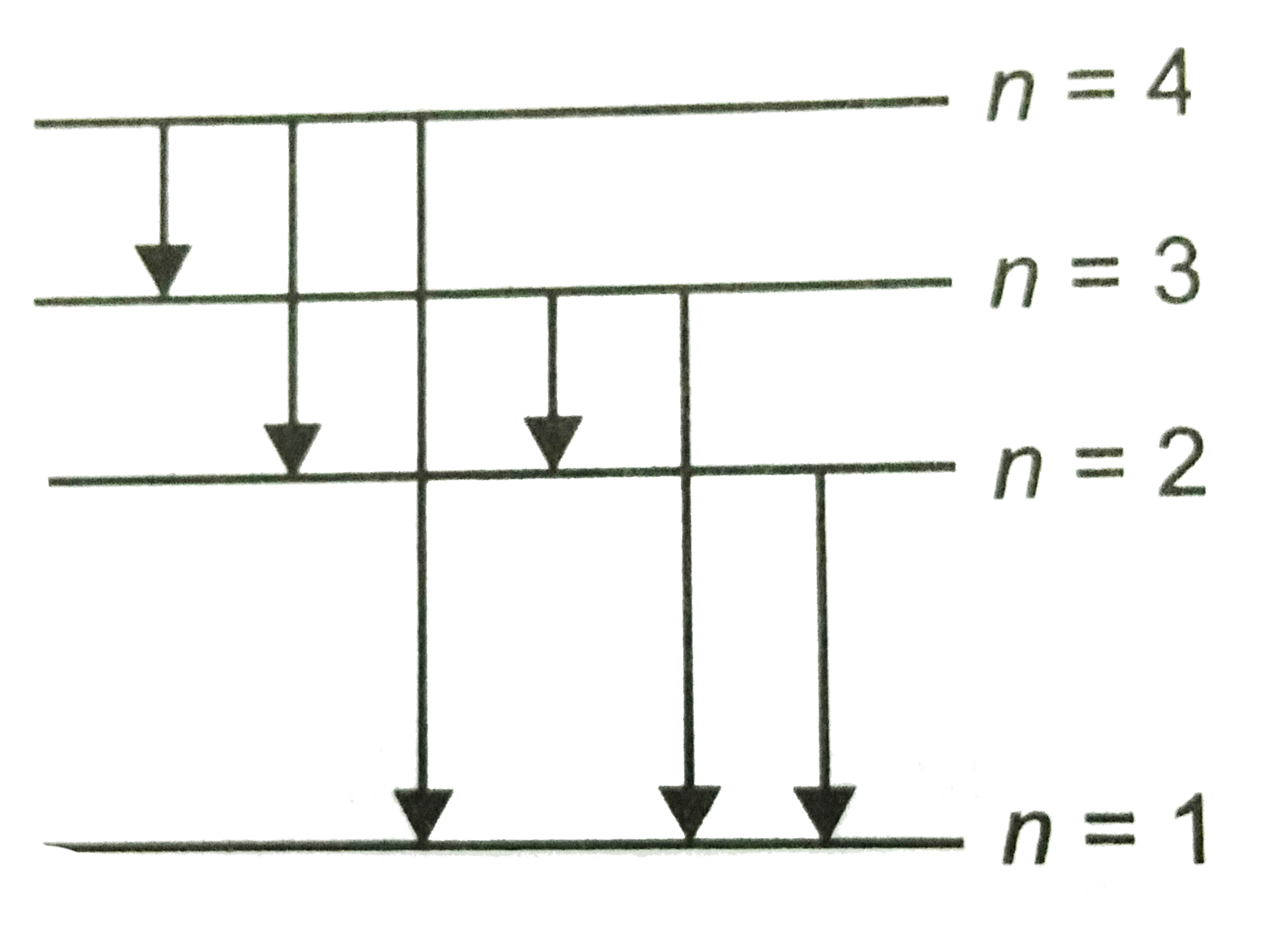
45 the figure is an energy-level diagram for a simple atom. (figure 1)
PDF Energy Changes Q1. - Weebly (a) Complete the reaction profile in Figure 1. Draw labelled arrows to show: • The energy given out (ΔH) • The activation energy. Figure 1 (3) (b) When ethene reacts with bromine, energy is required to break covalent bonds in the molecules. Explain how a covalent bond holds two atoms together. (2) (Figure 1) is an energy-level diagram for a simple atom ... Below is both an energy level diagram for an atom and an emission spectrum for that atom. Match the peak in the emission spectrum to the corresponding transition in the energy level diagram. III Intensity n = 1 (nm) O A Peaki=e, Peak II = f, Peak III = C, Peak IV = b O B Peak I=b, Peak II = c Peak III = f, Peak IV = e O C Peak I = a, Peak II = d...
Figure 2. Schematic diagram of the energy levels of carbon ... The self-absorption technique is a simple method to determine the line integrated density of metastable atoms in low-pressure plasmas. In order to employ this technique, it is necessary to make an...

The figure is an energy-level diagram for a simple atom. (figure 1)
Semiconductor Electronic: Material, Devices And Simple ... Jul 02, 2019 · ρ ~ 10 11. 10 19 Ωm, σ ~ 10-11. 10-19 Sm-1 4. Energy Band In a crystal due to interatomic interaction, valence electrons of one atom are shared by more than one atom in the crystal. Now, splitting of energy level takes place. The collection of these closely spaced energy levels are called an energy band. 5. PDF This question is about the reaction of ethene and bromine ... The students used the apparatus shown in the diagram below. € In one experiment the temperature of 50 g of water increased from 22.0 °C to 38.4 °C. The mass of alcohol burned was 0.8 g. Calculate the heat energy (Q) in joules, released by burning 0.8 g of the alcohol. Nitrogen energy-level diagram - Big Chemical Encyclopedia Nitrogen energy-level diagram Draw simple molecular orbital energy-level diagrams to indicate how the bonding in the saline hydrides, such as NaH or KH, differs from that between hydrogen and a light p- block element such as carbon or nitrogen. [Pg.741] Figure 6.21 Energy level diagram for nitrogen/oxygen.
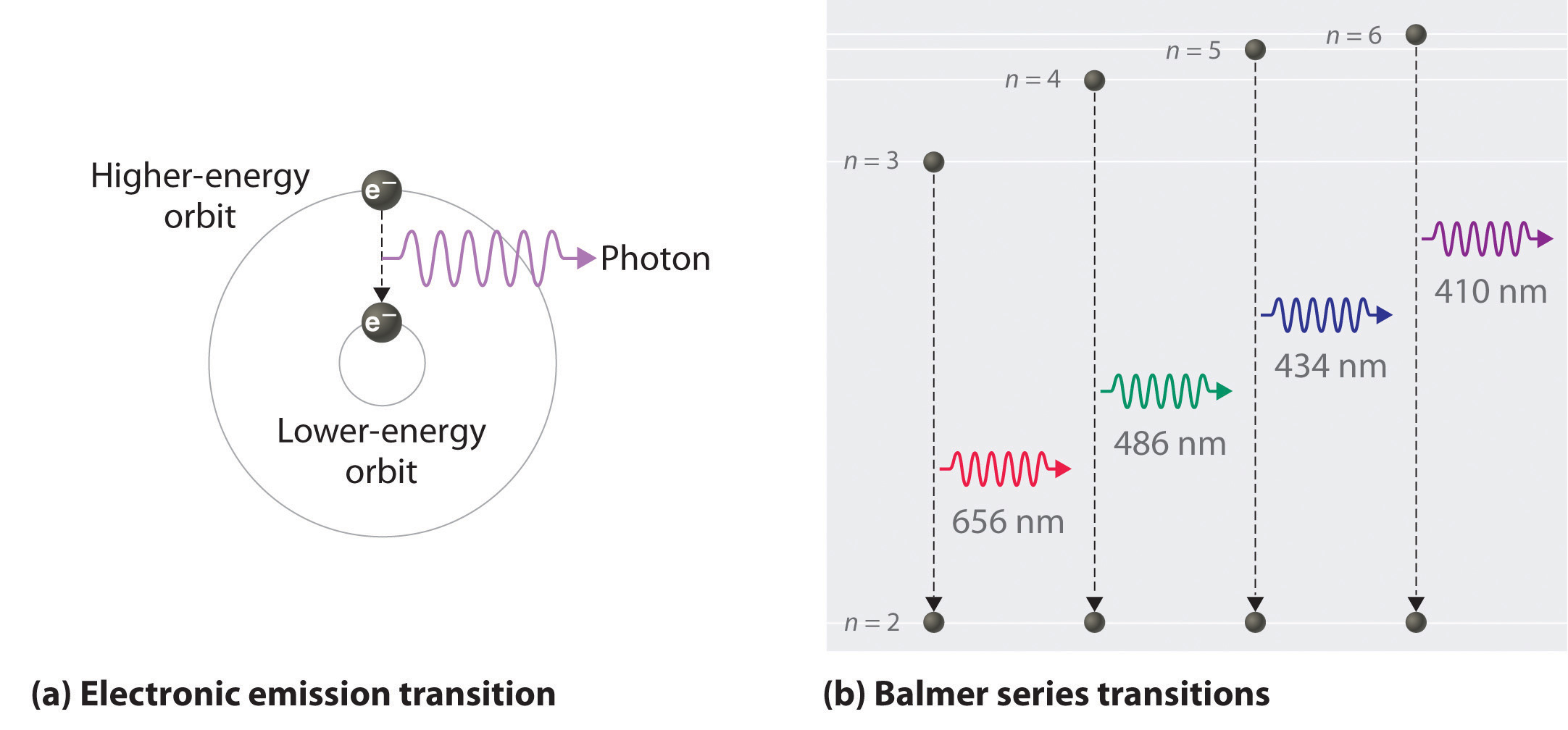
The figure is an energy-level diagram for a simple atom. (figure 1). Diagram Independent variable Observation 5 b The students ... • heated the water by burning propane for 1 minute • measured the temperature of the water again. The student found the temperature change was 20 °C. The student can calculate the energy released, in joules (J), using the equation: energy released (J) = mass of water (g) × 4.2 × temperature change (°C) (i) Use the student's result to calculate the energy released in joules (J). EXPERIMENT 2 -10 ABSORPTION OF BETA AND GAMMA RAYS from an atom due to excited electrons, with the notable difference that the MeV energy changes of the nucleus are approximately 106 times as large as energy changes in transitions between atomic states (where ∆E atomic ≈ several eV). See Figure 1. Figure 1. Typical Nuclear Energy Level Diagram. Solved (Figure 1) is an energy-level diagram for a simple Transcribed image text: (Figure 1) is an energy-level diagram for a simple atom. What wavelengths, in mm, appear in the atom's emission spectrum? How do you calculate the energy of an electron in the ... You can calculate the ground state energy using The Bohr Model A simple expression for the energy of an electron in the hydrogen atom is: E=-(13.6)/(n^2) where the energy is in electron volts n is the principle quantum number. This gives rise to the familiar electron energy level diagram where they converge and coalesce. So for an electron in n=1: E=-13.6"eV" To convert to joules you can x ...
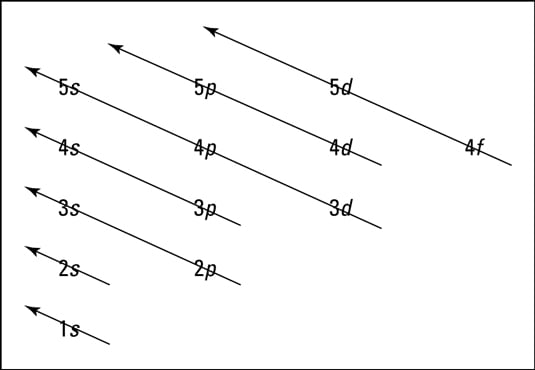
Energy Level Diagram - Different Energy Shells Around the ... Below is a blank energy level diagram which helps you depict electrons for any specific atom. At energy level 2, there are both s and p orbitals. The 2s has lower energy when compared to 2p. The three dashes in 2p subshells represent the same energy. 4s has lower energy when compared to 3d. Therefore, the order of energy level is as follows: s ... Bohr’s Theory of the Hydrogen Atom | Physics Figure 1. Niels Bohr, Danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. His many contributions to the development of atomic physics and quantum mechanics, his personal influence on many students and colleagues, and his personal integrity, especially in the face of Nazi oppression, earned him a prominent place in history. The energy levels of a hypothetical atom are shown below ... In figure `E_(1) and E_(2)` represent some of the energy levels of the hydrogen atom. Which one of the following transitions produced a photon of wave asked May 30, 2019 in Physics by AnushkaYadav ( 88.9k points) Solved (Figure 1) is an energy-level diagram for a simple Transcribed image text: (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum?
Solved The figure (Figure 1) is an energy-level diagram for Question: The figure (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? a.) 31 b.) ... The energy level diagram for an hydrogen like atom is ... The energy level diagram for an hydrogen like atom is shown in the figure.The radius of its first Bohr orbit is 0 eV n = ∞ - 6.04 eV n = 3 - 13.6 eV n = 2 - 54.4 eV n = 1 CLASSES AND TRENDING CHAPTER class 5 The Fish Tale Across the Wall Tenths and HundredthsParts and Whole Can you see the Pattern? class 6 Phys. Rev. A 92, 013807 (2015) - Three-photon coherence in ... Figure 1 (a) Simplified schematic diagram for the TPEIA experiment. (b) Energy-level diagram for the 5 S 1 / 2 − 5 P 3 / 2 − 5 D 5 / 2 transition in Rb 87 atoms. The two coupling beams are linearly polarized in (c) parallel or (d) perpendicular direction. Reuse & Permissions Solved (Figure 1) is an energy-level diagram for a simple Question: (Figure 1) is an energy-level diagram for a simple atom. Part A What wavelengths appear in the atom's emission spectrum?
Phonon - Wikipedia In physics, a phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids.Often referred to as a quasiparticle, it is an excited state in the quantum mechanical quantization of the modes of vibrations for elastic structures of interacting particles.
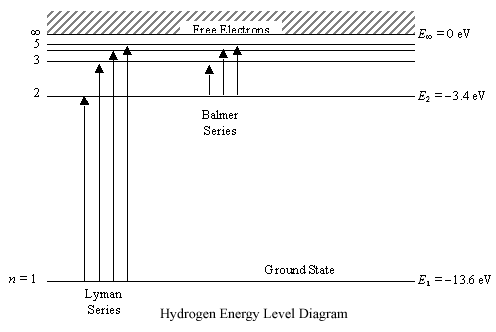
Bohr's Theory of the Hydrogen Atom | Physics II Figure 5. An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 and E 2.
Solved (Figure 1) is an energy-level diagram for a simple Enter your answers in ascending order separated by commas. lambda = 310, 500, 830 nm What wavelengths appear in the atom's absorption spectrum? Enter your ...
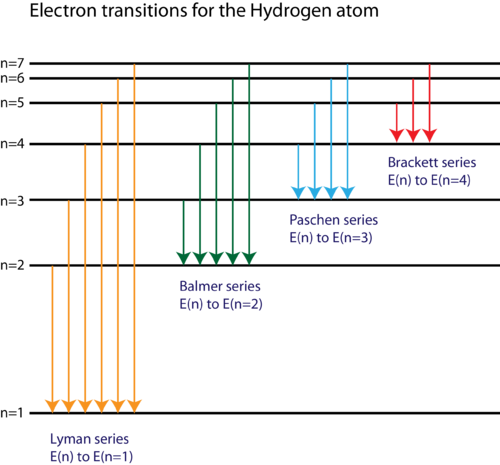
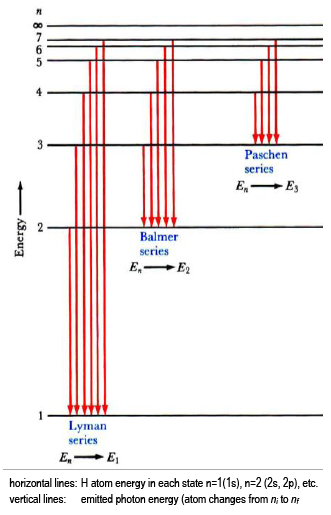
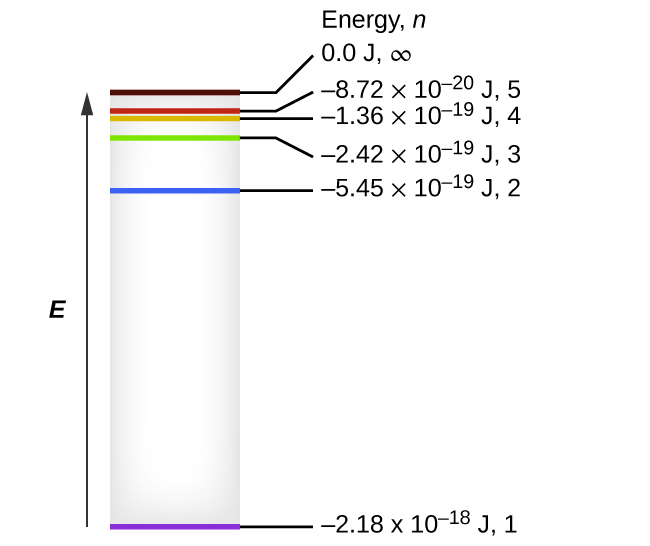
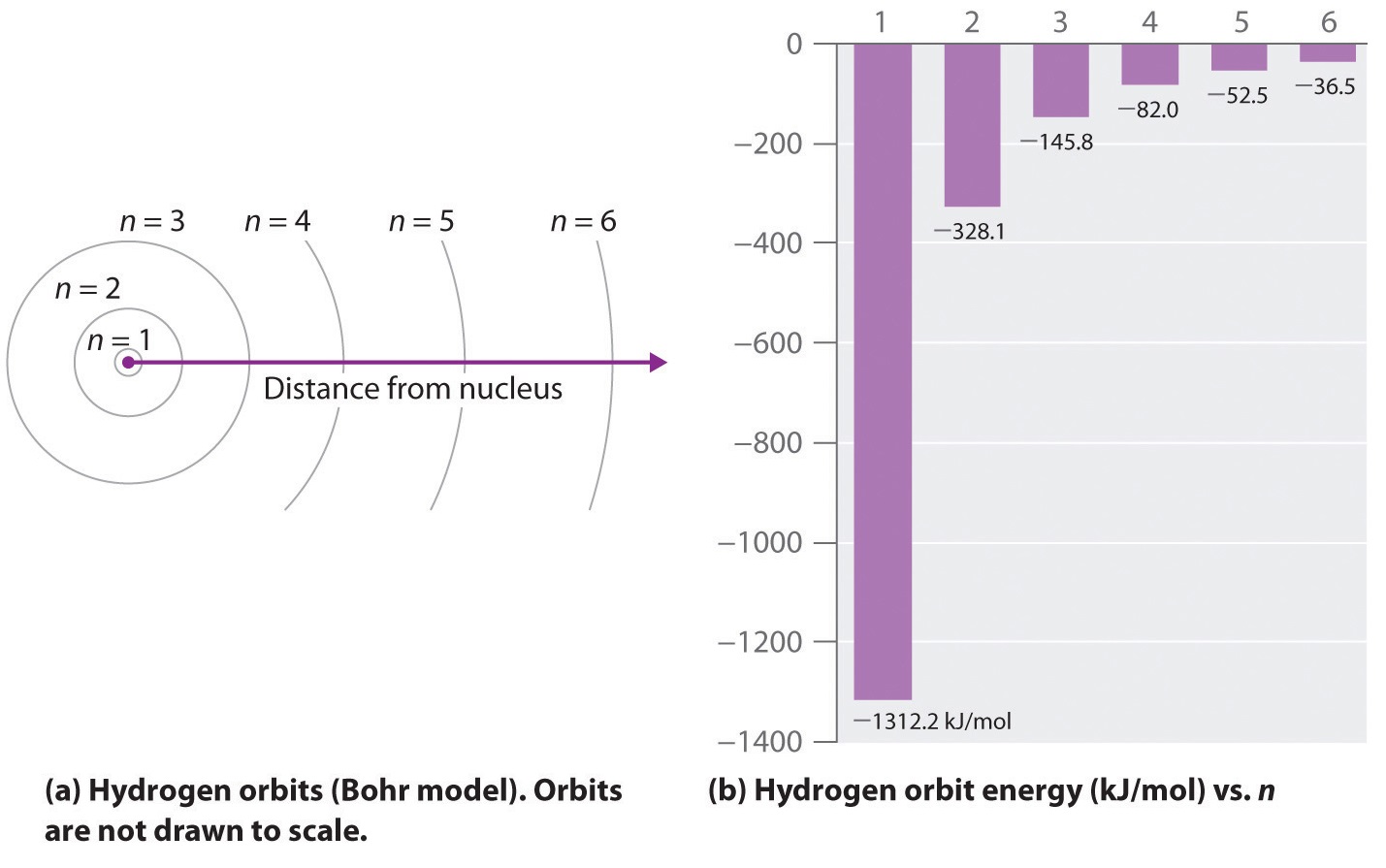
Energy Level Diagram For Hydrogen - Mini Physics The ionization energy of an atom is the energy required to remove the electron completely from the atom. (transition from ground state n = 0 to infinity n = ∞ ). For hydrogen, the ionization energy = 13.6eV. When an excited electron returns to a lower level, it loses an exact amount of energy by emitting a photon.
Carbon energy level diagram - Big Chemical Encyclopedia Draw simple molecular orbital energy-level diagrams to indicate how the bonding in the saline hydrides, such as NaH or KH, differs from that between hydrogen and a light p- block element such as carbon or nitrogen. [Pg.741] These equations lead to the energy values E = a + ft and E = a — ft.
SOLVED:Figure shows the energy-level diagram for neon. (a ... Figure shows the energy-level diagram for neon. (a) Verify that the energy of the photon emitted when neon goes from its metastable state to the one immediately below is equal to 1.96 e V. (b) Show that the wavelength of this radiation is 633 n m. (c) What wavelength is emitted when the neon makes a direct transition to its ground state? Answer
In the given figure, the energy levels of hydrogen atom ... >> In the given figure, the energy levels o Question In the given figure, the energy levels of hydrogen atom have been shown along with some transitions marked A, B, C, D and E. The transitions A , B and C respectively represent: A The ionization potential of hydrogen, second member of Balmer series and third member of Paschen series. B
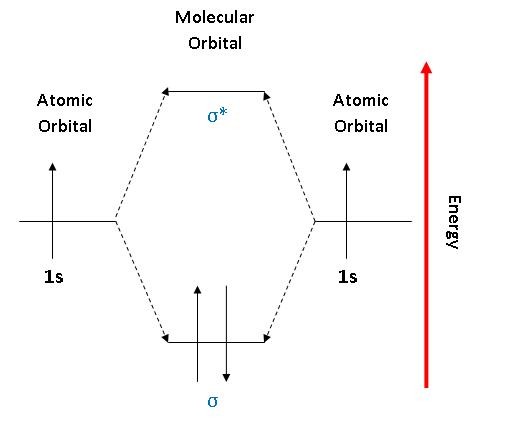
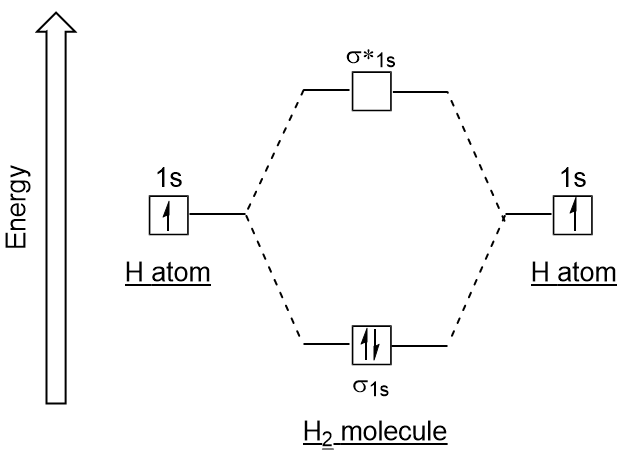
Benzene Lewis Structure, Molecular Geometry, Hybridization ... Apr 14, 2022 · Now, let us have a look at the MO diagram of benzene. The p-orbitals on each carbon atom could overlap to form six molecular orbitals, three bonding orbitals (ψ1 to ψ3) and three antibonding orbitals (ψ4 to ψ6) as shown in the figure. The bonding MO ψ1 is of lowest energy and most stable containing all six carbon atoms, i.e; it is delocalized.
22.1 The Structure of the Atom - Physics | OpenStax Figure 22.10 An energy-level diagram plots energy vertically and is useful in visualizing the energy states of a system and the transitions between them. This diagram is for the hydrogen-atom electrons, showing a transition between two orbits having energies E 4 E 4 and E 2 E 2. The energy transition results in a Balmer series line in an ...
PDF Temperature Dependent Lifetime Measurements of ... A simple energy level diagram in Figure 7 serves as the basis for obtaining a simple rate equation illustrating: (1) that the fluorescence is characterized by single exponential decay, and (2) the temperature dependence of this decay. It is assumed that the state is excited instantaneously with a population of N0. The change in number of ...
Solved (Figure 1) is an energy-level diagram for a simple ... Expert Answer 100% (3 ratings) Transcribed image text: (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? Enter your answers in ascending order separated by commas. What wavelengths appear in the atom's absorption spectrum? Enter your answers in ascending order separated by commas.
1. Semiconductor Materials & Physics Figure 1.2 exhibits three cubic-crystal unit cells - simple cubic, body-centered cubic, and face-centered cubic. The element semiconductors, silicon and germanium, have a diamond lattice structure as shown in Figure 1.3. This configuration belongs to the cubic-crystal family and can be envisaged as two interpenetrating fcc sublattices with
Atomic Energy Levels (video) - Khan Academy We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV.
The figure is an energy-level diagram for a simple atom. For this question consider the figure to the right that shows an energy level diagram for a certain atom (not hydrogen). Several transitions are shown and labeled by letters. Note the diagram is not drawn to scale.
PDF AP PHYSICS 2011 SCORING GUIDELINES - College Board For the correct answer 1 point DE=¥4.8 10 J-19 Note: This point is also awarded for an answer consistent with the energy-level diagram shown in part (a). ii. 1 point For selecting "Visible Light" only 1 point The wavelength for the nn=Æ=21 transition is found in part (b). l
PDF GCSE Grade O(l) This is the method used. 1. Measure 50 cm 3 hydrochloric acid into a glass beaker. 2. Measure the temperature of the hydrochloric acid. 3. Measure a given mass of potassium hydrogencarbonate. 4. Add the potassium hydrogencarbonate to the hydrochloric acid. 5. Stir until all the potassium hydrogencarbonate has reacted. 6.
4.10: Second-Row Diatomic Molecules - Chemistry LibreTexts Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence electrons (7 from each F atom), all of the energy levels except the highest, are filled. This diagram shows 8 electrons in bonding orbitals and 6 in antibonding orbitals, resulting in a bond order of 1.
Schematic diagram of the energy levels of aluminium atom ... download scientific diagram | schematic diagram of the energy levels of aluminium atom and the emission lines of 2 s 1 / 2 → 2 p o 3 / 2 (396.15 nm) and from publication: a novel method of using...
CHAPTER 10 AUGER ELECTRON SPECTROSCOPY The Auger process for a solid is schematically illustrated in Figure 10.2. The KL 2L 3 Auger transition, illustrated in this diagram, involving ionization, (1) A core-electron in the atom is removed by the high-energy incident electronically excited ion (ionization). (2) An electron from the L 2 in a
Solved The figure is an energy-level diagram for a simple ... The figure is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum? #1: From wavelength 3 to 1. #2: From wavelength 3 to 2. #3: From wavelength 2 to 1. What wavelengths appear in the atom's absorption spectrum?
Solved (Figure 1) is an energy-level diagram for a simple | Chegg.com Transcribed image text: (Figure 1) is an energy-level diagram for a simple atom. What wavelengths appear in the atom's emission spectrum?
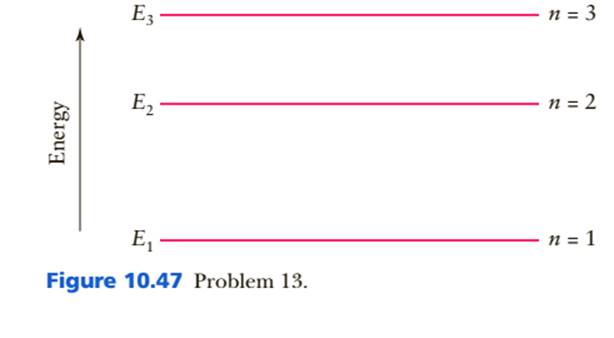
An energy-level diagram for a hypothetical atom is shown ... An energy-level diagram for a hypothetical atom is shown above. a. Determine the frequency of the lowest energy photon that could ionize the atom, initially in its ground state. b. Assume the atom has been excited to the state at -1.0 electron volt. i. Determine the wavelength of the photon for each possible spontaneous transition. ii.
Solved (Figure 1) is an energy-level diagram for a simple Question: (Figure 1) is an energy-level diagram for a simple atom. Part A What wavelengths appear in the atom's emission spectrum?
Nitrogen energy-level diagram - Big Chemical Encyclopedia Nitrogen energy-level diagram Draw simple molecular orbital energy-level diagrams to indicate how the bonding in the saline hydrides, such as NaH or KH, differs from that between hydrogen and a light p- block element such as carbon or nitrogen. [Pg.741] Figure 6.21 Energy level diagram for nitrogen/oxygen.
PDF This question is about the reaction of ethene and bromine ... The students used the apparatus shown in the diagram below. € In one experiment the temperature of 50 g of water increased from 22.0 °C to 38.4 °C. The mass of alcohol burned was 0.8 g. Calculate the heat energy (Q) in joules, released by burning 0.8 g of the alcohol.
Semiconductor Electronic: Material, Devices And Simple ... Jul 02, 2019 · ρ ~ 10 11. 10 19 Ωm, σ ~ 10-11. 10-19 Sm-1 4. Energy Band In a crystal due to interatomic interaction, valence electrons of one atom are shared by more than one atom in the crystal. Now, splitting of energy level takes place. The collection of these closely spaced energy levels are called an energy band. 5.

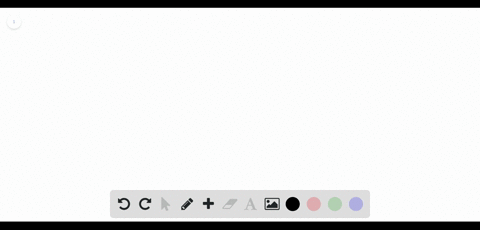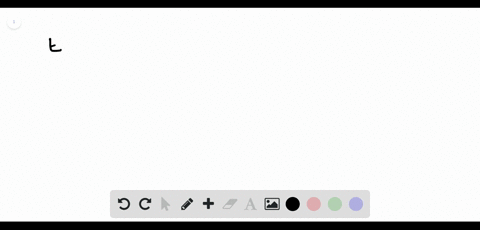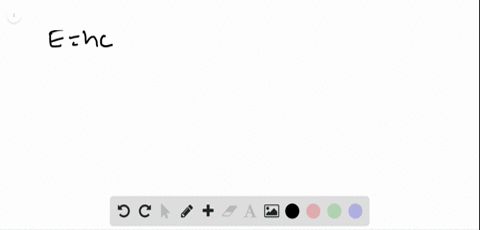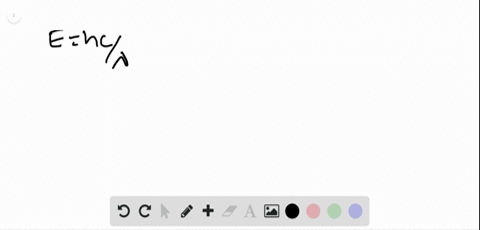















0 Response to "45 the figure is an energy-level diagram for a simple atom. (figure 1)"
Post a Comment